Trans. Nonferrous Met. Soc. China 23(2013) 3793-3798
A method for calculation of ion distribution in reaction system forming hydroxide
Zhe-nan JIN1, Dae-rok JONG1,2, Jong-su HONG1,3, Yong-hun JONG1,2, Dian-kun LU1, Guo-bao CHEN1
1. Key Laboratory of Ecological Utilization of Multi-metal Intergrown Ores of Ministry of Education, Northeastern University, Shenyang 110819, China;
2. Department of Metallurgical Science and Engineering, Chongjin Mine & Metallurgy University, Chongjin 999091, DPR of Korea;
3. Department of Metallurgical Science and Engineering, Kimchaek University of Technology, Pyongyang 999093, DPR of Korea
Received 24 April 2013; accepted 9 August 2013
Abstract: A formula was proposed to calculate the distribution of metal ions quantitatively in chemical reaction system forming hydroxide where precipitation and complex are formed together. The effects of some factors on formation of precipitation and complex were investigated, and the corresponding precipitation rates of zinc, iron (III), aluminum, copper and magnesium were calculated. As a result, it shows that the proposed formula is reliable. By the proposed formula, the existence state of metal ions in hydroxides reaction system with any metal ions can be well described and the effects of some factors on the distribution of metal ions were determined.
Key words: hydroxide; metal ions distribution; precipitation rate; complex forming rate
1 Introduction
In non ferrous metallurgy, hydroxide precipitate method is commonly used to recover valuable metals and purify the leaching solution usually [1-7]. For example, magnesium hydroxide and calcium hydroxide are used to form hydroxides precipitates to recover valuable metals such as nickel, cobalt, cadmium, copper, zinc and chrome from leaching solution or wastewater [1-5]. In zinc hydrometallurgy, zinc calcine is leached by sulfuric acid solution first, and then it is put into the leaching solution to neutralize in order to separate impure metals by hydroxides precipitation method [6,7]. In addition, hydroxide precipitation method is used in the manufacture of nano-sized metal hydroxides, e.g. nano-sized copper hydroxide and magnesium hydroxide [8-10], and complex metal hydroxides materials, e.g. rare earth (Pr, Nd, Sm, Eu, Gd, etc.) complex metal hydroxide, and Ni(II)-Cr(III) double hydroxides [11-13]. Except for the alkali metals and another few metals, most metal hydroxide solution contain precipitates (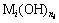 ) and complex ions (
) and complex ions (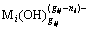 ) [14-16]. Therefore, the quantitative description and reasonable explanation for the distribution of metal ions in this complex metal hydroxide solution system have an important significance for scientific research and industrial production.
) [14-16]. Therefore, the quantitative description and reasonable explanation for the distribution of metal ions in this complex metal hydroxide solution system have an important significance for scientific research and industrial production.
Research on chemical reactions in hydroxide solution system has been reported [1,5,14,15], but it is restricted to precipitation reaction system 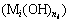 or coordination reaction system (
or coordination reaction system (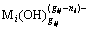 ) in the single component. Therefore, few works were reported on the multi-component chemical reaction system containing both precipitate
) in the single component. Therefore, few works were reported on the multi-component chemical reaction system containing both precipitate 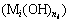 and complex (
and complex (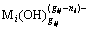 ).
).
Based on the quantitative study on the chemical reaction in the above solution system, a reaction model and a formula were proposed, which are suitable for the multi-component chemical reaction system containing both precipitate 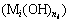 and complex (
and complex (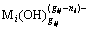 ). According to the calculation results, the distribution of metal ions is analyzed in the multi-component solution containing zinc, iron (III), aluminum, copper and magnesium and pH changes with the increase of the total alkali concentration. Furthermore, the calculated results and experimental results are compared.
). According to the calculation results, the distribution of metal ions is analyzed in the multi-component solution containing zinc, iron (III), aluminum, copper and magnesium and pH changes with the increase of the total alkali concentration. Furthermore, the calculated results and experimental results are compared.
2 Multi-component hydroxide reaction system mode
When certain amount of alkali is added to multi-component solution containing several metal ions, the metal ions (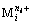 ) and hydroxide ion (OH-) will react and form precipitate
) and hydroxide ion (OH-) will react and form precipitate 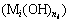 and complex (
and complex (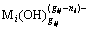 ).
).
Reaction system model is shown in Fig. 1, where 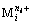 represents any metal ions in solution (i=1, 2, …, p), Yk represents the complexing agent, while the solid line represents main reaction and the dotted line represents side reaction.
represents any metal ions in solution (i=1, 2, …, p), Yk represents the complexing agent, while the solid line represents main reaction and the dotted line represents side reaction.
3 Theoretical derivation of formula
The precipitation rate (G) of metal cations is defined as the ratio of the concentration of metal cation used to form hydroxide precipitation (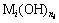 ) and the total concentration of the metal cation, expressed as
) and the total concentration of the metal cation, expressed as
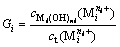 (i=1, 2, …, p) (1)
(i=1, 2, …, p) (1)
where 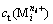 represents the total concentration of any metal cations in solution,
represents the total concentration of any metal cations in solution, 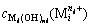 represents the concentration of metal cation (
represents the concentration of metal cation (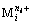 ) used to form hydroxide precipitation
) used to form hydroxide precipitation 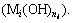 The concentration of metal cation (
The concentration of metal cation (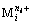 ) that reacts with hydroxide ion (OH-) to form hydroxide precipitation (
) that reacts with hydroxide ion (OH-) to form hydroxide precipitation (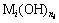 ) is given by formula (2).
) is given by formula (2).
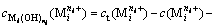
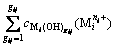 (2)
(2)
where 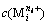 represents the concentration of residual free metal cation remaining in solution after metal cation (
represents the concentration of residual free metal cation remaining in solution after metal cation (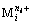 ) reacting with hydroxide ion (OH-) to form precipitation and complex; gij represents the maximum coordination number of complex formed by metal cation (
) reacting with hydroxide ion (OH-) to form precipitation and complex; gij represents the maximum coordination number of complex formed by metal cation (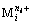 ) and hydroxide ion (OH-).
) and hydroxide ion (OH-).
The concentration 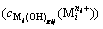 of complex
of complex 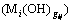 formed by metal cation
formed by metal cation 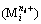 and hydroxide ion (OH-) is calculated by
and hydroxide ion (OH-) is calculated by
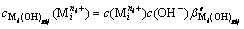
(i=1, 2, …, p; gij=1, 2, …, gij) (3)
where c(OH-) represents the concentration of residual hydroxide ion (OH-) remaining in solution after metal cation (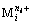 ) reacting with hydroxide ion (OH-) to form precipitation and complex;
) reacting with hydroxide ion (OH-) to form precipitation and complex; 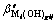 represents the conditional accumulative stability constants of complex
represents the conditional accumulative stability constants of complex 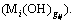
Deriving formula (1) to formula (3) gests formula (4):
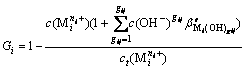
(i=1, 2, …, p) (4)
The concentration of residual metal cation remaining in solution after the reaction with hydroxide ion (OH-) is expressed by conditional solubility product constant formula [1,4,16,17] as follows:
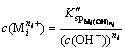 (i=1, 2, …, p) (5)
(i=1, 2, …, p) (5)
The sedimentation rate of any metal cations is obtained by substituting formula (5) into formula (1):
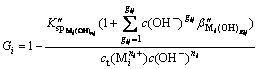
(i=1, 2, …, p) (6)
The formation rate of complex 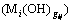 is expressed by formula (7) as follows:
is expressed by formula (7) as follows:
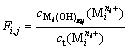
(i=1, 2, …, p; gij=1, 2, …, gij) (7)
Formulas (3) and (4) are deformed first and then substituted into formula (7), then,
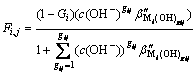
(i=1, 2, …, p; gij=1, 2, …, gij) (8)
According to formulas (6) and (8), in order to get
the sedimentation rate (Gi) of precipitate (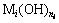 ) and the formation rate of complex (
) and the formation rate of complex (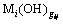 ), the concentration c(OH-) of residual hydroxide ion (OH-) remaining in solution after metal cation (
), the concentration c(OH-) of residual hydroxide ion (OH-) remaining in solution after metal cation (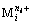 ) reacting with hydroxide ion (OH-) to form precipitation and complex must be obtained.
) reacting with hydroxide ion (OH-) to form precipitation and complex must be obtained.
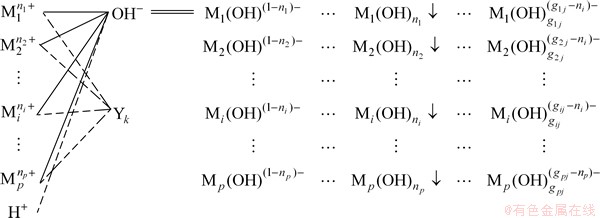
Fig. 1 Model of metal ion distribution when alkali is added to multi-component solution
The total concentration of hydroxide ion (OH-) in the reaction system was defined as ct(OH-), then,
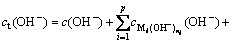
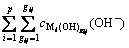 (9)
(9)
where 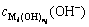 represents the concentration of hydroxide ion (OH-) that reacts with metal cation (
represents the concentration of hydroxide ion (OH-) that reacts with metal cation (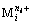 ) to form precipitate;
) to form precipitate; 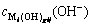 represents the concentration of hydroxide ion (OH-) that reacts with metal cation (
represents the concentration of hydroxide ion (OH-) that reacts with metal cation (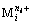 ) to form complex.
) to form complex.
According to formulas (1) and (7), 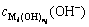 and
and 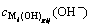 can be expressed as
can be expressed as
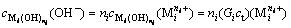
(i=1, 2, …, p) (10)
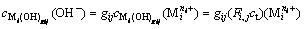
(i=1, 2, …, p; gij=1, 2, …, gij) (11)
Formulas (6), (8), (10) and (11) are substituted into formula (9), then,
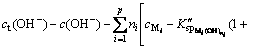
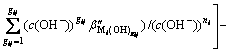
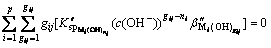 (12)
(12)
4 Result and discussion
Solution components and their concentrations used in the experiment are listed in Table 1. The water for the experiment was deionized water.
Table 1 Components and concentrations of mixed solution
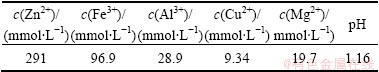
The precipitation rates of zinc, iron, aluminum, copper and magnesium were determined as follows. 0.5 L solution of metal ions was prepared firstly and its component is listed in Table 1. Then 1.06 mol/L sodium hydroxide solution was added and stirred for 30 min. After that the mixture was placed and held for 2 h. When the precipitation process was finished, the concentrations of metallic cations remaining in the solution were determined by flame atomic absorption spectrophotometry (AAS). The instruments used in AAS are as follows: WFX1F2B2 atomic absorption spectrophotometer (Beijing Second Optical Instrument Factory), XWT-S desk type recorder (Shanghai Automation Instrumentation Third Factory), magnesium, iron, zinc, aluminum, copper element hollow cathode lamp (Shanghai Optoelectronic Components Factory).
When alkali was added to the experimental solution whose components are shown in Table 1. The effects of alkaline quantities on the distribution of metal cation in the solution and the pH change are shown in Fig. 2. The data about the solubility product of metal hydroxides precipitation and cumulative stability constant of metal hydroxide complex are from literatures [1,5,14-16].
From Fig. 2(a), the zinc ion (Zn2+) transformed into precipitate (Zn(OH)2) gradually with the increase of ct(OH-). When ct(OH-) was 0.974 mol/L, the sedimentation rate could surpass 99%. When ct(OH-) was higher than 1.03 mol/L, precipitate transformed into complex ion 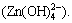 When ct(OH-) was 1.76 mol/L, the sedimentation rate reduced to below 1.13%, the formation rate of complex ion
When ct(OH-) was 1.76 mol/L, the sedimentation rate reduced to below 1.13%, the formation rate of complex ion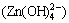 could surpass 98.6%, the formation rate of complex ion
could surpass 98.6%, the formation rate of complex ion 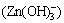 was as low as 0.21%, and complex ion (Zn(OH)+) almost did not exist.
was as low as 0.21%, and complex ion (Zn(OH)+) almost did not exist.
Figure 2(b) shows that Fe3+ existed in the form of metal cation Fe3+ (about 81%) and complex ion Fe(OH)2+ (about 19%) in the solution first. After alkali was added to solution, Fe3+ formed precipitate Fe(OH)3 gradually, while complex ion 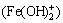 almost did not exist. When the concentration of alkali reached above 0.292 mol/L, the sedimentation rate could surpass 99.99%.
almost did not exist. When the concentration of alkali reached above 0.292 mol/L, the sedimentation rate could surpass 99.99%.
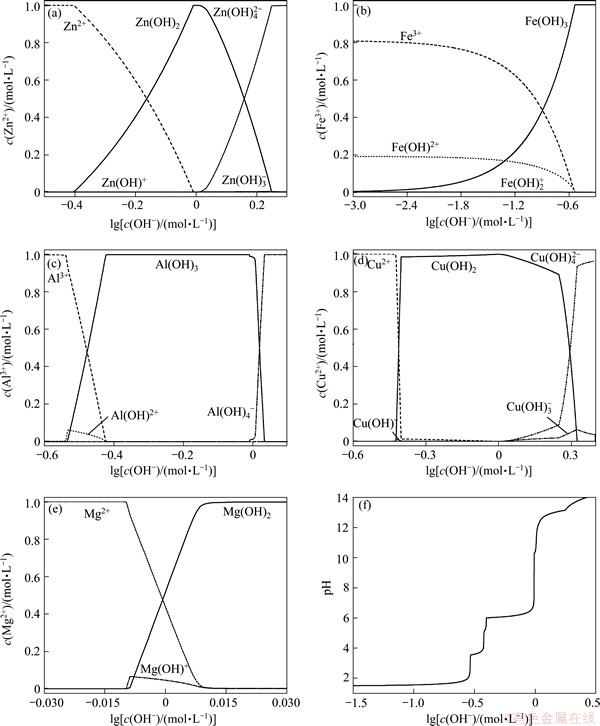
Fig. 2 Influence of total alkali concentration on distribution of Zn2+ (a), Fe3+ (b), Al3+ (c), Cu2+ (d), Mg2+ (e) and pH (f)
From Fig. 2(c), aluminium ion(Al3+) transformed into complex ion (Al(OH)2+) and precipitated (Al(OH)3) gradually with the increase of ct(OH-). But the formation rate of complex ion (Al(OH)2+) was not large, even when ct(OH-) was 0.294 mol/L, it reached 6.20%. When ct(OH-) increased continuously, the formation rate of complex ion (Al(OH)2+) reduced and sedimentation rate increased. When ct(OH-) was 0.378 mol/L, the sedimentation rate could surpass 99.99%. When ct(OH-) increased further, precipitate began to dissolve and form complex ion 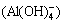 . When the alkali concentration reached 1.08 mol/L, precipitate was completely dissolved in the form of complex ion
. When the alkali concentration reached 1.08 mol/L, precipitate was completely dissolved in the form of complex ion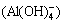 .
.
Figure 2(d) shows that copper ion (Cu2+) transformed into complex ion (Cu(OH)+) and precipitate (Cu(OH)2) gradually with the increase of ct(OH-). But the formation rate of complex ion (Cu(OH)+) was not large, even when ct(OH-) was 0.376 mol/L, it reached 1.47%. When ct(OH-) increased continuously, the formation rate of complex ion (Cu(OH)+) reduced and sedimentation rate increased. When ct(OH-) was 0.570 mol/L, the sedimentation rate could surpass 99.99%. When ct(OH-) increased further, precipitate began to dissolve and form complex ion 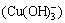 and complex ion
and complex ion 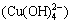 . But the formation rate of complex ion
. But the formation rate of complex ion 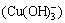 was not large, even when ct(OH-) was 2.11 mol/L, it reached 6.38%. With the increase of ct(OH-), the formation rate of complex ion
was not large, even when ct(OH-) was 2.11 mol/L, it reached 6.38%. With the increase of ct(OH-), the formation rate of complex ion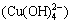 increased, when ct(OH-) was 2.00 mol/L, it could reach 55.60%.
increased, when ct(OH-) was 2.00 mol/L, it could reach 55.60%.
From Fig. 2(e), magnesium ion (Mg2+) transformed into complex ion (Mg(OH)+) gradually with the increase of ct(OH-). When ct(OH-) was 0.980 mol/L, the formation rate of complex ion (Mg(OH)+) reached 6.56%. When ct(OH-) increased continuously, the formation rate of complex ion (Mg(OH)+) reduced and sedimentation rate increased. When ct(OH-) was 1.02 mol/L, the sedimentation rate of Mg(OH)2 could surpass 99%.
Figure 2(f) shows that when alkali was added to solution, pH of the solution varied complicatedly. With the increase of ct(OH-), pH increased slowly. When ct(OH-) was 0.282 mol/L, pH reached 2.07, which was 0.91 higher than the initial pH 1.16. Then ct(OH-) increased, pH increased rapidly. When ct(OH-) was 0.294 mol/L, pH reached 3.56. When ct(OH-) was 0.370 mol/L, pH increased slowly (pH=3.92). When ct(OH-) increased continuously, pH increased rapidly. As ct(OH-) was 0.370 mol/L, pH reached 6.02. As ct(OH-) increased, pH increased gradually. When ct(OH-) was 0.974 mol/L, pH reached 7.09. When ct(OH-) increased continuously, pH increased rapidly. As ct(OH-) was 1.04 mol/L, pH reached 11.90. At this point, when ct(OH-) was 9.84 mol/L (pH=10.3), a inflection point occurred. When ct(OH-) increased, pH increased faster. As ct(OH-) was 2 mol/L, pH reached 13.56.
The inflection points in some curves in Fig. 2 were related to the formation of precipitate and complex ion by the reaction of metal cation and hydroxide ion. Therefore, with the increase of ct(OH-), if the concentration of any component of the solution changed, the existence form (sedimentation rate and formation rate of complex ion) of other components and the value of pH changed.
The reason that the distribution of metal cation in chemical reaction system containing both hydroxide precipitate and complex ion is that the ability (solubility product of hydroxide precipitate and cumulative stability constant of hydroxide complex ion) of forming precipitate and complex ion by the reaction of metal cation and hydroxide ion and their concentration is different. That is to say, the formation amount of precipitate and complex ion changes with the change of species and concentration of metal cation.
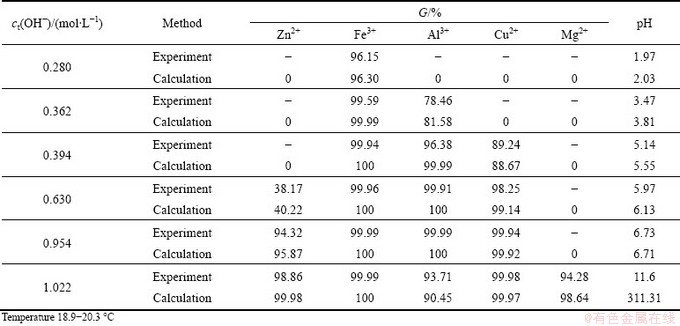
When alkali is added to solution, the sedimentation rate of Zn2+, Fe3+, Al3+, Cu2+, Mg2+ and pH change are calculated. The comparison of calculated values and experimental values is listed in Table 2. The comparative result proves the reliability of the proposed formula.
Based on the above formula, the maximum ct(OH-) corresponding to the sedimentation of metal cation and pH of the solution can be obtained when alkali is added to multi-component solution containing some metal cations.
Table 2 Comparison of calculation result and experimental values of precipitation rates for Zn2+, Fe3+, Al3+, Cu2+, Mg2+ and pH
5 Conclusions
1) The distribution of metal cation in multi-component hydroxide reaction system is well described and the influence of several factors (the amount of alkali, cation concentration) on sedimentation rate and formation rate of complex ion is investigated quantitatively.
2) The distribution of metal cation and pH change are complicated when alkali is added to multi-component solution containing Zn2+, Fe3+, Al3+, Cu2+ and Mg2+.
3) The calculated values based on proposed formulas are in accordance with the experimental values significantly.
References
[1] LIU Jian-hua, HONG De-en, PAN Yong, ZHU Chao-han. Methods of precipitating cobalt from cobalt-containing solution [J]. Hydrometallurgy of China, 2008, 27(3): 151-153. (in Chinese)
[2] HARVEY R, HANNAH R, VAUGHAN J. Selective precipitation of mixed nickel–cobalt hydroxide [J]. Hydrometallurgy, 2011, 105(3-4): 222-228.
[3] TIAN Jun, YI Jing-qun, SHEN Kai-hong, RAO Guo-hua, JIANG Min-tao. Solution chemistry on precipitate-flotation of rare earth from leach liquor of the weathered crust elution-deposited rare earth ore [J]. Chinese Rare Earths, 2011, 32(4): 1-6. (in Chinese)
[4] TAN Hao-qiang, WU Wei, LIU Zhi-bin, HE Wen-jie, HAN Hong-da, MA Yue. Features of removal cadmium by chemical precipitation method [J]. Water Technology, 2010, 4(4): 10-12. (in Chinese)
[5] LIU Ding-fu. Studies on separation of Fe3+ from electroplating waste water [J]. Journal of Guizhou University of Technology: Natural Science Edition, 2007, 36(6): 91-93. (in Chinese)
[6] DENG Zhi-ming, ZHOU Zheng-hua. Studies on the precipitation of iron in the leaching process of zinc production via RLE [J]. Hunan Nonferrous Metals, 2002, 18(1): 23-26. (in Chinese)
[7] ZHAI Xiu-jing. Heavy metal metallurgy [M]. Beijing: Metallurgical Industry Press, 2011: 74-340. (in Chinese)
[8] LI Guang, YUAN Ying-cai, LI Xi-meng. Preparation of nanopowder Cu(OH)2 by the action of high intensity ultrasonic field and its characteristic [J]. Journal of Beijing Institute of Graphic Communication, 2005, 13(1): 26-28. (in Chinese)
[9] JIANG Wen-jun, HUA Xiao, HAN Qiao-feng. Preparation of lamellar magnesium hydroxide nanoparticles via precipitation method [J]. Powder Technology, 2009, 191(3): 227-230.
[10] WANG Pei-pei, LI Cai-hong, GONG Hai-yan, WANG Hong-qiang, LIU Jian-rong. Morphology control and growth mechanism of magnesium hydroxide nanoparticles via a simple wet precipitation method [J]. Ceramics International, 2011, 37(8): 3365-3370.
[11] XINA Ying, WANG Zhong-ping, QI Yong-xin. Synthesis of rare earth (Pr, Nd, Sm, Eu and Gd) hydroxide and oxide nanorods (nanobundles) by a widely applicable precipitation route [J]. Journal of Alloys and Compounds, 2010, 507(1): 105-111.
[12] OZAWA M, ONOE R, KATO H. Formation and decomposition of some rare earth (RE=La, Ce, Pr) hydroxides and oxides by homogeneous precipitation [J]. Journal of Alloys and Compounds, 2006, 408(9): 556-559.
[13]  M, BLESA M A, REGAZZONI A E. Homogeneous precipitation of layered Ni(II)–Cr(III) double hydroxides [J]. Journal of Colloid and Interface Science, 2007, 309(1): 72-77.
M, BLESA M A, REGAZZONI A E. Homogeneous precipitation of layered Ni(II)–Cr(III) double hydroxides [J]. Journal of Colloid and Interface Science, 2007, 309(1): 72-77.
[14] LI Guang-ping, MA Zhong-ge, YAN Feng. The study of the best result of pH created by amphoteric hydroxide [J]. Journal of Jinzhou Normal College: Natural Science Edition, 2002, 23(1): 27-28. (in Chinese)
[15] ROGER J M, RUSSELL J M. Characterisation and humidity-sensing properties of aluminium(oxy)-hydroxide films prepared by cathodically induced precipitation [J]. Sensors and Actuators B, 2007, 128(1): 124-132.
[16] YU Wen-qin. Chemical analysis [M]. Beijing: Science Press, 2008: 25-186. (in Chinese).
氢氧化物反应体系中离子分布的计算方法
金哲男1,郑大录1,2,洪正秀1,3, 郑英勋1,2, 路殿坤1, 陈国宝1
1. 东北大学 多金属共生矿生态利用教育部重点实验室 沈阳 110819;
2. 清津矿山金属大学,清津999091,朝鲜;
3. 金策工业综合大学,平壤 999093,朝鲜
摘 要:提出一种计算公式,定量地计算同时形成氢氧化物沉淀和氢氧配合物的化学反应体系中金属离子的分布,考察影响氢氧化物沉淀和氢氧配合物形成的各种因素;基于提出的公式,计算锌、铁、铝、铜和镁的沉淀率,理论计算与实验结果一致。根据提出的计算公式,可以很好地描述在任意金属离子同时存在的氢氧化物反应体系中金属离子的存在形态,考察影响金属离子分布的因素。
关键词:氢氧化物;金属离子分布;沉淀率;配合物形成率
(Edited by Xiang-qun LI)
Foundation item: Project (51304047) supported by the National Natural Science Foundation of China; Project (20131037) supported by Science and Technology Foundation of Liaoning Province, China
Corresponding author: Zhe-nan JIN; Tel: +86-24-83681319; E-mail: jinzn@smm.neu.edu.cn
DOI: 10.1016/S1003-6326(13)62931-3