
Fabrication of CTP/HAp novel gradient composite bioceramics
SHEN Mei-ling(申美玲), ZHAO Zhong-wei(赵中伟), WEN Shi-mei(文士美),
CHEN Xing-yu(陈星宇), LI Hong-gui(李洪桂)
School of Metallurgical Science and Engineering, Central South University, Changsha 410083, China
Received 15 July 2007; accepted 10 September 2007
Abstract: Calcium-titanium-phosphate(CaTi4(PO4)6, CTP)/hydroxyapatite(HAp) is a kind of novel gradient composite bioceramics, which has excellent biocompatibility and bioactivity. CTP ceramic film was synthesized one-step on the surface of titanium using micro-arc oxidation(MAO). The CTP/HAp composite bioceramics were prepared by soaking CTP film in HAp inducing solution for several days. XRD, SEM and EDX were used to characterize the bio-ceramic films phase and composition, morphology and component. The influence of electrolyte molar ratio of Ca to P and the current density to the synthesis of film was studied, and the optimized value of parameters above were 1/6 and 15A/dm2. The parameters of HAp inducing solution, such as component and pH value were also studied and the best pH value which is adjusted by NaOH is 6.4.
Key words: micro-arc oxidation; CaTi4(PO4)6; hydroxyapatite; bioceramic; deposit
1 Introduction
Calcium-phosphate biomaterials possessed similar chemical components and physical properties with the bone tissue of human[1-3], which also had excellent biocompatibility and good bone bonding ability. Therefore, it was widely used in medical field[4]. Among those calcium-phosphate biomaterials, hydroxyapatite (HAp) was most widely used as implants materials due to its excellent osteoinductor and bioactive in clinical medicine[5-6]. However, the pure HAp was a kind of brittleness material, which had poor toughness, lower intensity, weak plasticity and cohesion. So it can’t be used as implants material directly. Some researchers[7] showed calcium-titanium-phosphate(CaTi4(PO4)6, CTP) was a kind of novel bioceramic material with good bioactivity, suited coefficient of thermal expansion with Ti substrate and stable chemical properties.
Some methods had been used to prepare this kind of CTP ceramic such as plasma spraying[8], thermal spraying and hydrothermal reaction at high temperature and high pressure[9]. But these methods had some shortcomings such as complex process and manipulation. Micro-arc oxidation(MAO), also named as plasma electrolytic oxidation or anodic spark oxidation[10], which had a particularly interesting process, and can produce a porous, relatively rough, and firmly adherent oxide film on valve metals such as Al, Ti, Mg and Zr. Then, MAO was widely used to make the surface treatment of metal[11]. Various kinds of components of MAO ceramic can be obtained by adjusting the composition of electrolyte. So it is possible to prepare the CTP ceramic film on the Ti substrate.
Therefore, according to the design idea[12] of functional gradient material, it was very interesting to deposit HAp coating on the surface of CTP ceramic. The novel CTP/HAp gradient composite bioceramic material would be prepared. This kind of gradient composite bioceramic would possess all the advantages of metal and ceramic materials. It not only had high intensity and good toughness of metal but also had excellent bioactivity. At the same time, the bonding intensity[13] between the coating with Ti substrate can be improved. Till now, no literature was reported to prepare the CTP/HAp gradient composite bioceramic by this method.
In this study, the MAO technique was used to prepare the CTP ceramic coating on the Ti substrate in the electrolyte of Ca and P solution. By soaking it in the HAp inducing solution, the CTP/HAp gradient composite bioceramic was obtained successfully. 2 Experimental
2.1 Micro-arc oxidation
All titanium anode sample (10 mm×10 mm×0.1 mm) used in MAO treatments were commercially pure titanium. Before using, it was polished by 360 waterproof abrasive paper and W2801 metallographic abrasive paper, then rinsed by ethanol and distilled water successively. For the electrolytes, the concentration of hexametaphosphate was 25 g/L, while the molar ratio of Ca to P and current density were various.
A DC voltage stabilized current stabilized power (YJ901 type 0-600 V/0-0.5 A, Huguang Instrument Co. Shanghai, China) was used to supply the current of micro-arc oxidation. After coating, the samples were thoroughly rinsed with distilled water and then dried in the air.
2.2 HAp induction
The HAp inducing solution was prepared by dissolving analytical reagent grade chemicals of CaCl2, NaH2PO4 and NaHCO3 in distilled water. The concentrations of CaCl2 and NaH2PO4 were both 5×10-3 mol/L, and that of NaHCO3 was changed among 3×10-3, 4.5×10-3 and 6×10-3 mol/L. Each of the sample after MAO procedure was soaked in 250 mL of the HAp inducing solution kept at 37 ℃ in a single oscillatory cycle instrument (HZ-921S Hualida, Science-Tech equipment Taicang Jiangsu) with 100 r/min. The HAp inducing solution was changed every 2 d.
2.3 Surface characterization
The phase and composition of the coating were analyzed by X-ray diffraction(XRD). The morphology of the coating was characterized by scanning electron microscopy (SEM, JEOL, JSM-6360LV). The component of coating was measured by energy disperse X-ray spectrometric microanalyzer (EDX-GENESIS 60S, EDAX Company of America).
3 Results and discussion
3.1 Effect of MAO parameters on CTP ceramic film
3.1.1 Effect of molar ratio of Ca to P of electrolyte
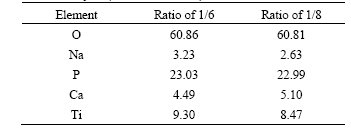
The phases and composition of CTP ceramic prepared in different concentrations of  and different molar ratios of Ca to P in electrolytes are shown in Fig.1. It can be seen that the diffraction peaks of CaTi4(PO4)6, anatase-TiO2, rutile-TiO2 and Ti increases with the increasing concentration of
and different molar ratios of Ca to P in electrolytes are shown in Fig.1. It can be seen that the diffraction peaks of CaTi4(PO4)6, anatase-TiO2, rutile-TiO2 and Ti increases with the increasing concentration of 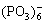 and molar ratio of Ca to P (from c to b); the increase is much evident when the concentration of
and molar ratio of Ca to P (from c to b); the increase is much evident when the concentration of 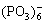 is fixed and the molar ratio of Ca to P is different(from b to a ). The phenomena from experiment show that, when both of the concentrations of
is fixed and the molar ratio of Ca to P is different(from b to a ). The phenomena from experiment show that, when both of the concentrations of  and molar ratio of Ca to P are higher, the spark lingers on the edge of sample as the CTP films haven’t formed completely. So the edge becomes thicker than the center.
and molar ratio of Ca to P are higher, the spark lingers on the edge of sample as the CTP films haven’t formed completely. So the edge becomes thicker than the center.
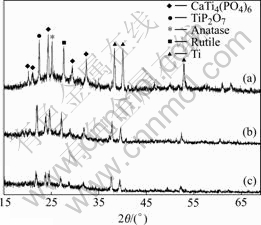
Fig.1 XRD patterns of CTP ceramic prepared in different concentrations of  and different molar ratios of Ca to P in electrolytes: (a) 25 g/L, 1/6; (b) 25 g/L, 1/8; (c) 20 g/L, 1/6
and different molar ratios of Ca to P in electrolytes: (a) 25 g/L, 1/6; (b) 25 g/L, 1/8; (c) 20 g/L, 1/6
The morphologies of CTP ceramic prepared in different concentrations of  and different molar ratios of Ca to P in electrolytes are shown in Fig.2. From Fig.2(c), some spherical pores with all kinds of diameters are not well separated and distributed inhomogeneously over the samples. Around the porous microstructures, some fused substance piled each other can be observed. In Fig.2(b), there are some smaller micro pores and less fused substance than that of Fig.2(c). Under the condition of 25 g/L of
and different molar ratios of Ca to P in electrolytes are shown in Fig.2. From Fig.2(c), some spherical pores with all kinds of diameters are not well separated and distributed inhomogeneously over the samples. Around the porous microstructures, some fused substance piled each other can be observed. In Fig.2(b), there are some smaller micro pores and less fused substance than that of Fig.2(c). Under the condition of 25 g/L of  and 1/6 molar ratio of Ca to P, the micro pores and fused substance are distributed homogeneously (Fig.2(a)).
and 1/6 molar ratio of Ca to P, the micro pores and fused substance are distributed homogeneously (Fig.2(a)).
All above indicate that the concentration of  has some effects on the crystallization degree of CTP phase. Then ,a better crystallization can be obtained under a optimized concentration of
has some effects on the crystallization degree of CTP phase. Then ,a better crystallization can be obtained under a optimized concentration of  . Although higher concentration of Ca2+ can also obtain better crystallization of CTP, it is not beneficial to the homogeneity of phases on CTP films.
. Although higher concentration of Ca2+ can also obtain better crystallization of CTP, it is not beneficial to the homogeneity of phases on CTP films.
The component of CTP ceramic prepared in the electrolytes with same concentration of  and different molar ratios of Ca to P are listed in Table 1. The effects of Ca2+ is investigated during MAO in this experiment.
and different molar ratios of Ca to P are listed in Table 1. The effects of Ca2+ is investigated during MAO in this experiment.
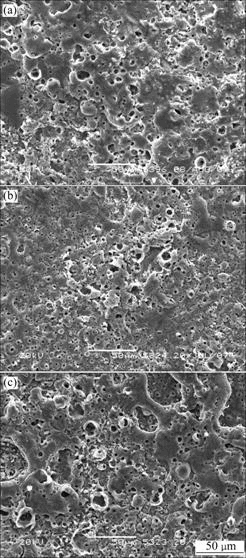
Fig.2 SEM images of CTP ceramic prepared in different concentrations of and different ratios of Ca to P in electrolytes: (a) 25 g/L, 1/6; (b) 25 g/L, 1/8; (c) 20 g/L, 1/6
and different ratios of Ca to P in electrolytes: (a) 25 g/L, 1/6; (b) 25 g/L, 1/8; (c) 20 g/L, 1/6
Table 1 EDX chemical composition of CTP ceramic prepared in same concentration of  and different ratios of Ca to P in electrolytes (molar fraction, %)
and different ratios of Ca to P in electrolytes (molar fraction, %)
From the table 1, we can see that the elements of O, P and Ti on the MAO film are higher and the elements of Na and Ca are lower when the concentration of Ca2+ is higher. The results of molar ratio of Ca to P from Table 1 are 1/5.1 and 1/4.5, respectively. That’s to say, when the content of Ca2+ in electrolyte increase, the molar ratio of Ca to P of MAO films decreases. These indicate the increment of Ca2+ incorporates into the ceramic films in the form of amorphous calcium- titanium oxide, such as CaTiO3, not the CTP. The increase of Ca2+ can improve the electrical conduction of electrolyte and lead to fierce spark discharge on a local ceramic film, which can result in the inhomogeneity of CTP ceramic films.
3.1.2 Effect of current density
Under the condition of 25 g/L of  and 1/6 of molar ratio of Ca to P, the XRD patterns of CTP ceramic prepared at different applied current densities by MAO are shown in Fig.3. From the figure, when the current density is 15 A/dm2, the XRD intensity of new formed phase, such as CTP and TiO2, becomes the strongest, and that of Ti is the weakest. While the current density increases or decreases, the XRD intensity of new phase decreases. These indicate when the current density is too high or low, it doesn’t have positive effects on the CTP ceramic. As the current density is too low, the Ti substrate cannot be micro-arc oxidized into new phase completely. At the same time, the reaction time becomes long. While the current density is too high, the reaction of Ti substrate with electrolyte is too fast, which can lead the poor crystallization degree of CTP phase.
and 1/6 of molar ratio of Ca to P, the XRD patterns of CTP ceramic prepared at different applied current densities by MAO are shown in Fig.3. From the figure, when the current density is 15 A/dm2, the XRD intensity of new formed phase, such as CTP and TiO2, becomes the strongest, and that of Ti is the weakest. While the current density increases or decreases, the XRD intensity of new phase decreases. These indicate when the current density is too high or low, it doesn’t have positive effects on the CTP ceramic. As the current density is too low, the Ti substrate cannot be micro-arc oxidized into new phase completely. At the same time, the reaction time becomes long. While the current density is too high, the reaction of Ti substrate with electrolyte is too fast, which can lead the poor crystallization degree of CTP phase.
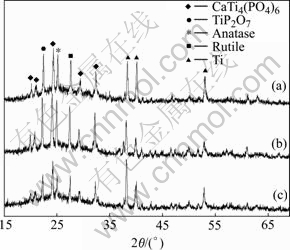
Fig.3 XRD patterns of CTP ceramic prepared in different current densities: (a) 10 A/dm2; (b) 15 A/dm2; (c) 20 A/dm2
3.2 Deposit process of HAp inducing
3.2.1 Effect of pH of HAp inducing solution
The HAp inducing solution is prepared according to the method described as section 2.2, and its pH value is adjusted to 6.40, 6.50 and 6.60 with the 0.04 mol/L NaOH. After soaking in HAp inducing solution for 6 d, the XRD patterns and SEM images of sample are shown in Fig.4 and Fig.5, respectively.
From Fig.4, as pH is 6.4, the XRD pattern of sample
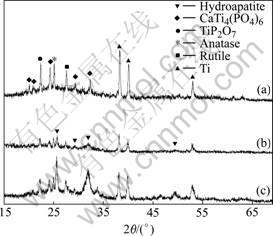
Fig.4 XRD patterns of ceramic after soaked at different pH values inducing solution: (a) 6.4; (b) 6.5; (c) 6.6
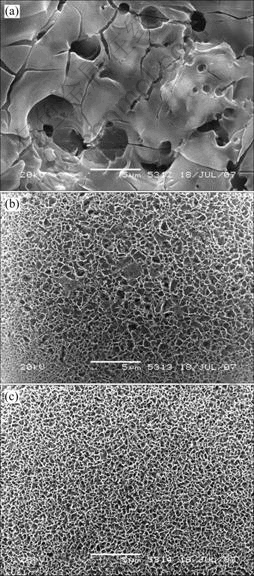
Fig.5 SEM images of ceramic after soaked at different pH values inducing solution: (a) 6.4; (b) 6.5; (c) 6.6
is similar to that of the sample without being soaked in HAp inducing solution (Fig.1 and Fig.2). Some micro-porous structures connected with each other and some cracks formed after rapid cooling can be observed. And those structures are the typical morphologies formed by MAO[14]. These show there is no HAp coating on the surface. When the pH value is more than 6.4, some apparent HAp can be detected. With increasing pH value, the intensity of HAp becomes stronger. From Fig.5(c), some sheet structural HAp can be observed. Comparing Fig.5(b) with Fig.5(c), the surface structures are more intact, more uniform and denser.
3.2.2 Effect of composition of HAp inducing solution
The HAp inducing solution is prepared according to the method described as section 2.2, and its pH value is adjusted with NaHCO3. The pH values are adjusted to 6.80, 6.60 and 6.40, respectively. After soaking in HAp inducing solution for 6 d, the XRD pattern of sample is shown in Fig.6. From Fig.6, the bioactive substance, HAp, can be detected by XRD. But its intensities are all weaker than those of Fig.4. The possible reason is that the alkali of NaHCO3 is weaker than that of NaOH. So NaHCO3 cannot provide enough OH- to form HAp.
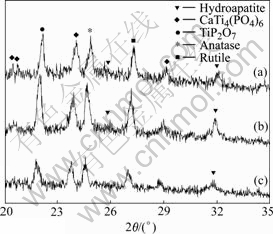
Fig.6 XRD patterns of samples soaked in HAp inducing solution with different pH values adjusted with NaHCO3: (a) 6.8; (b) 6.6; (c) 6.4
3.2.3 Mechanism of inducing HAp deposition
After soaking in HAp inducing solution, HAp coating can be deposited on the CTP ceramic films prepared by MAO. And the CTP/HAp gradient composite bioceramics can be obtained. The reason which can induce HAp to deposit is related to the surface composition and structure of CTP films. The bioceramic prepared by MAO contains CTP, TiO2 and some amorphous CaTiO3, which plays a very important role in inducing HAp to deposit.
Firstly, the main component, CaTi4(PO4)6, of ceramic contains the element of Ca and P which are exsited in bone tissue. So the surface of CTP ceramic can be the location of HAp deposited easily. And then the CTP ceramic possesses good affinity for HAp. As it soaks in HAp inducing solution, the contact angle between ceramic surface and HAp is decreased, accordingly, the value of f(θ) diminishes too. The decrease of f(θ) value[15] can result in the decrease of energy that is needed for forming the critical nucleus size of HAp, so the HAp nucleus forms more easily.
Secondly, the TiO2 phase of ceramic has lower point of zero electric charge, e.g. anatase is 6.2 and rutile is 5.0[16]. When the ceramic soaks in HAp inducing solution, its surface can adsorb some OH- and 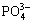 and is positively charged. The surface with positive charge can adsorb Ca2+ by Coulomb force. After the reaction of TiO2 with solution, a great amount of Ca2+, OH- and
and is positively charged. The surface with positive charge can adsorb Ca2+ by Coulomb force. After the reaction of TiO2 with solution, a great amount of Ca2+, OH- and 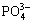 are enriched on the surface of ceramic films.
are enriched on the surface of ceramic films.
In addition, the amorphous CaTiO3 of ceramic is easily hydrolyzed in HAp inducing solution, some Ca2+, OH- and hydration TiO(OH)2 are produced. The Ti-OH radical existed on the ceramic surface provides advantage for the forming of HAp nucleus[15,17].
Under the reaction of surface composition with HAp inducing solution, more and more Ca2+, OH- and 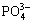 are enriched on the surface of ceramic films, and their concentration are very high. With the soaking time increasing slowly, the concentrations of Ca2+, OH- and
are enriched on the surface of ceramic films, and their concentration are very high. With the soaking time increasing slowly, the concentrations of Ca2+, OH- and 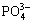 become higher, which can cause the super- saturation of HAp increasing. Once the super- saturation exceeds the critical level required for nucleation of HAp, the HAp crystal deposits on the ceramic films (Fig.7). Those ions react each other according to the following reaction:
become higher, which can cause the super- saturation of HAp increasing. Once the super- saturation exceeds the critical level required for nucleation of HAp, the HAp crystal deposits on the ceramic films (Fig.7). Those ions react each other according to the following reaction:
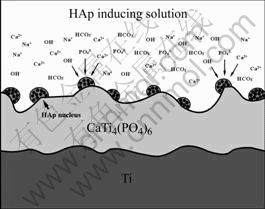
Fig.7 HAp forming process in HAp inducing solution
10Ca2++6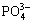 +2OH-= Ca10(PO4)6(OH)2(HAp)
+2OH-= Ca10(PO4)6(OH)2(HAp)
CTP ceramic films prepared by MAO possess the rough and micro-porous structure, which not only benefit the deposition of HAp but also can improve the bond strength between HAp coating and CTP ceramic.
4 Conclusions
1) During the process of preparation of CTP ceramic, the concentration of has some effects on the crystallization degree of CTP phase; the higher concentration of Ca2+ is harmful to the homogeneity of all phases in the ceramic films; the current density has no apparent effects on the ceramic films, but it can’t be applied too high. So the CTP ceramic can be prepared by MAO under the condition of 25 g/L of
has some effects on the crystallization degree of CTP phase; the higher concentration of Ca2+ is harmful to the homogeneity of all phases in the ceramic films; the current density has no apparent effects on the ceramic films, but it can’t be applied too high. So the CTP ceramic can be prepared by MAO under the condition of 25 g/L of , 1/6 of molar ratio of Ca to P and 15 A/dm2 of current density.
, 1/6 of molar ratio of Ca to P and 15 A/dm2 of current density.
2) In the process of inducing HAp, the HAp coating can be obtained on a certain pH value adjusted with NaOH in HAp inducing solution; when the pH value is adjusted with NaHCO3, the crystal structure of HAp is not as good as the former. The CTP, TiO2 and some amorphous CaTiO3 of ceramic play a very important role in inducing HAp to deposit. So the HAp can be obtained by soaking CTP films in the inducing solution with 1/1 of molar ratio of Ca to P and 6.5 of pH value for 6 d.
References
[1] CHEN De-min. Physical properties of apatite bone cement [J]. J Biomed Eng, 2000, 17(1): 13-15. (in Chinese)
[2] LIU Ai-hong, SUN Kang-ning, ZHAO Ping. Research development of calcium phosphate cement [J]. Materials Review, 2005, 19(2): 17-19. (in Chinese)
[3] DUAN You-rong, YAO Zhe, WANG Chao-yuan, CHEN Ji-yong, ZHANG Xing-dong. A study of bone-like apatite formation on porous calium phosphate ceramics in dynamic SBF [J]. J Biomed Eng, 2002, 19(3): 365-369. (in Chinese)
[4] LEE J H, KIM S E, KIM Y J, CHI C S, OH H J. Effects of microstructure of anodic titania on the formation of bioactive compounds [J]. Materials Chemistry and Physics, 2006(98): 39-43.
[5] ISHIZAWA H, OGINO M. Hydro thermal precipitation of hydroxyapatite on anodic titanium oxide films containing Ca and P [J]. Journal of Materials Science, 1999(34): 5893-5898.
[6] CHEN Xing-yu, ZHAO Zhong-wei, CHEN Ai-liang, LI Hong-gui. Pulsed electrodeposition of hydroxyapatite on titanium substrate in solution containing hydrogen peroxide [J]. Trans Nonferrous Met. Soc China, 2007, 17(3): 617-621.
[7] WEN Shi-mei, ZHAO Zhong-wei. Direct synthesis of CaTi4(PO4)6 bio-ceramic film by micro-arc oxidation [J]. Materials Science and Engineering of Powder Metallurgy, 2005, 10(3): 191-194. (in Chinese)
[8] TOPIC M, NTSOANE T, HEIMANN R B. Microstructural characterisation and stress determination in as-plasma sprayed and incubated bioconductive hydroxyapatite coatings [J]. Surface and Coatings Technology, 2006, 201: 3633-3641.
[9] HSU Y S, CHANG E, LIU H S. Growth of phosphate coating on titanium substrate by hydrothermal process [J]. Ceramics International, 1998, 24(1): 7-12.
[10] ZHONG Tao-sheng, JIANG Bai-ling, LI Jun-ming. Characteristics, applications and research direction of micro-arc oxidation technology [J]. Electroplating and Finishing, 2005, 24(6): 47-50. (in Chinese)
[11] WEI Da-qing, ZHOU Yu, JIA De-chang, WANG Ya-ming. Effect of heat treatment on the structure and in vitro bioactivity of microarc-oxidized(MAO) titania coatings containing Ca and P ions [J]. Surface and Coatings Technology, 2007, 201: 8723-8729.
[12] DAI Hao, ZHOU Rong, FAN Gang. Preparation of graded bioactive ceramic on titanium [J]. Jiangsu Metallurgy, 2006, 34(2): 18-21. (in Chinese)
[13] QUAN Ren-fu, YANG Di-sheng, WU Xiao-chun, WANG Hong-bin, LI Wei, MIAO Xu-dong. Preparation and biocompatibility of graded zirconia-hydroxyapatite composite bioceramic [J]. Acta Materiae Compositae Sinica, 2006, 23(3): 114-122. (in Chinese)
[14] SONG W H, JUN Y K, HAN Yang, HONG S H. Biomimetic apatite coatings on micro-arc oxidized titania [J]. Biomaterials, 2004, 25: 3341-3349.
[15] HUANG Ping, XU Ke-wei, HAN Yong. Porous titanium layer of containing calcium phosphate and its mechanism of bioactivity [J]. Journal of the Chinese Ceramic Society, 2004, 32(12): 1449-1452. (in Chinese)
[16] HAN Yong, XU Ke-wei. Structural characterization of micro-arc oxidation formed titanium dioxide films containing Ca and P [J]. Journal of Inorganic Materials, 2001,16(5): 951-956. (in Chinese)
[17] HAYAKAWA S, OSAKA A. Biomimetic deposition of calcium phosphates on oxides soaked in a simulated body fluid [J]. Journal of Non-Crystalline Solids, 2000, 263/264: 409-415.
(Edited by LI Xiang-qun)
Foundation item: Project(50674105) supported by the National Natural Science Foundation of China
Corresponding author: ZHAO Zhong-wei; Tel: +86-731-8830476; E-mail: zhaozw@mail.csu.edu.cn