Effects of surfactant on properties of MIM feedstock
来源期刊:中国有色金属学报(英文版)2007年第1期
论文作者:李益民 刘相权 罗丰华 岳建岭
文章页码:1 - 8
Key words:metal injection molding(MIM); feedstock; surfactant; binder
Abstract: Effects of the surfactant for improving the properties of MIM feedstock were investigated. Feedstocks were prepared by 17-4PH stainless steel(SS) powder and paraffin wax-based binder containing different contents of stearic acid(SA) as the surfactant. The viscosity of the feedstock decreases significantly when the SA is added. Besides, the wetting angle of the binder against the 17-4PH SS powder decreases greatly and the critical solid loading increases with the adding of the SA. Fourier transformation infrared spectroscopy(FTIR) analysis was used to prove the interaction between the SA and the 17-4PH SS powder. Chemical bonding is found on the surface of 17-4PH SS powder after mixing and it helps a lot to enhance the interacting force between the binder and the powder. Then an adsorbing model was adopted to estimate the least content of the surfactant that formed a monolayer adsorption on the mono-sized spherical powder (with smooth surface). The least content of the surfactant is calculated to be 0.19%. Whereas, the experiments indicate that about 5% is the optimal value to improve the properties of the feedstock. The reason may come from two aspects: firstly, the powders used in current experiment are not all mono-sized spheres and the coarse surface of the powder has a great effect on the adsorptive capacity of the powder; secondly, multilayer adsorption is likely to occur on the powder surface, which will also increase the adsorptive capacity.
基金信息:by Chinese National Excellent Dissertation Foundation
by Program for New Century Excellent Talents in University of China

LI Yi-min(李益民), LIU Xiang-quan(刘相权), LUO Feng-hua(罗丰华), YUE Jian-ling(岳建岭)
State Key Laboratory of Powder Metallurgy, Central South University, Changsha 410083, China
Received 30 April 2006; accepted 13 September 2006
Abstract: Effects of the surfactant for improving the properties of MIM feedstock were investigated. Feedstocks were prepared by 17-4PH stainless steel(SS) powder and paraffin wax-based binder containing different contents of stearic acid(SA) as the surfactant. The viscosity of the feedstock decreases significantly when the SA is added. Besides, the wetting angle of the binder against the 17-4PH SS powder decreases greatly and the critical solid loading increases with the adding of the SA. Fourier transformation infrared spectroscopy(FTIR) analysis was used to prove the interaction between the SA and the 17-4PH SS powder. Chemical bonding is found on the surface of 17-4PH SS powder after mixing and it helps a lot to enhance the interacting force between the binder and the powder. Then an adsorbing model was adopted to estimate the least content of the surfactant that formed a monolayer adsorption on the mono-sized spherical powder (with smooth surface). The least content of the surfactant is calculated to be 0.19%. Whereas, the experiments indicate that about 5% is the optimal value to improve the properties of the feedstock. The reason may come from two aspects: firstly, the powders used in current experiment are not all mono-sized spheres and the coarse surface of the powder has a great effect on the adsorptive capacity of the powder; secondly, multilayer adsorption is likely to occur on the powder surface, which will also increase the adsorptive capacity.
Key words: metal injection molding(MIM); feedstock; surfactant; binder
1 Introduction
Metal injection molding is a near-net shaping technology that combines the advantages of plastic injection molding and conventional powder metallurgy. It is a cost effective technology for fabrication of small intricate and precise parts in large quantities[1-3]. Four typical steps for the MIM process are mixing, injection molding, debinding and sintering. Initially, a binder with suitable formulation is mixed with the metal powder to form a feedstock. During molding, the feedstock is injection molded to produce green parts with required shapes. The molded parts then undergo a debinding step where the binder is removed. After debinding, the parts are subjected to a sintering step and final products with required properties are obtained[4-5]. In recent years, MIM has gained extensive popularity from the material science and the industrial field due to its preponderances in fabrication area.
The binder system used for MIM usually involves the components of polymers, plasticizers, waxes and surfactants. The formulation of the binder has received much attention in the Refs.[6-10] mainly because an ideal binder system for different powder is still impossible to attain. In order to improve the binder properties such as surface wetting, spreading, adsorption and binder strengthening, the surfactant is often added as an additive in the binder[11]. Generally, the surfactant is a low molecular component that consists of a functional group adhering to the powder surface and an oriented molecular chain extending into the binder. Therefore, the surfactant acts as a bridge between the binder and the powder due to its particular structure and it enhances stabilization of the powders when mechanical shearing during mixing process breaks them apart[12]. The surfactant is critically important to the MIM process as mentioned in the Refs.[13-20]. Generally speaking, as a lubricant, it can decrease friction force existed among the powder particles so as to decrease viscosity of the feedstock and improve the solid loading; as a dispersant, it makes the powder particles disperse more easily in the binder and improves homogeneity of the feedstock; as a plasticizer, it can improve mixing properties among the binder components. More importantly, the surfactant can effectively enhance the adhesion strength between the binder and the powder, improving the strength of green parts and offering a potential way to eliminate the structural defects. Therefore, the surfactant has important influence on controlling the quality of final products.
A part of scholars did some fundamental jobs about the surfactant on the properties of MIM feedstock. WENJEA et al[21-22] indicated that surfactant- dependent behavior is critically related to the adsorption affinity of the surfactant molecules and the adding of the surfactant prevented the particles from making a direct surface-to-surface contact. JOHNSON and MORRISON [23] found out that the surfactant adsorbed on the powder surface can reduce the viscosity of the slurry compared with the uncoated powders and the hydrocarbon chains of the surfactant can produce stable non-aqueous dispersions for steric stability. DOROSZKOWSKI and LAMBOURNE[24] showed that the dispersion properties depend largely on the molecular architecture of the surfactant when he studied the dispersibility of the oxide powder in the non-aqueous liquids. LIN and GERMAN[25] revealed that adsorption of organic surfactants on a powder surface is usually through hydrogen bonding; moreover, they pointed out that chemical adsorption can occur on the powder surface when they investigated the interaction between the surfactant and the alumina powder. NOVAK et al[26] implied that not only the activity and quality of the surfactant, but also the type of the bonds is of essential importance for the success of injection moulding. LI et al[27] indicated that surfactants no matter hydrophilic or hydrophobic could also decrease the wetting angle of the binder against the powder and improve the solid loading of the powder. QUACKENBUSH et al[28]and BATER [29] found out that an insufficient coverage of the surfactant onto the powder surface resulted in the formation of particulate networks and led to an increase in suspension viscosity that is not desired by manufactures.
Due to the important roles of the surfactant in the MIM process, a series of experiments were carried out to study effects of the surfactant for improving the properties of the MIM feedstock. The objects of current paper include three aspects: 1) to verify the influence of the SA for improving the rheologic behavior, wetting ability and increasing the critical solid loading; 2) to discuss the reasons of the SA in improving the properties for the MIM feedstock; 3) to estimate the least content of the SA in theory using adsorption model and make a discussion compared with practical value in the current experiments.
2 Experimental
2.1 Materials
17-4PH SS powders with d10=5 mm, d50=12 mm, d80=22 mm (gas atomized, spherical in shape) were used in the experiment. The powder apparent density and tap density were 3.92 g/cm3 and 4.70 g/cm3, respectively. The specific area of the powder was 0.11 g/cm2 measured by BET method (by means of nitrogen adsorption). Fig.1 shows the SEM photograph of the 17-4PH SS powders. The binder used in current investigation contained ethylene-vinyl acetate copolymer (EVA), paraffin wax(PW), microcrystal paraffin wax (MW), high density polyethylene(HDPE) and stearic acid(SA). Table 1 shows four compositions of the binder mixtures.

Fig.1 SEM photograph of 17-4PH SS powder
Table 1 Compositions of binder (mass fraction, %)
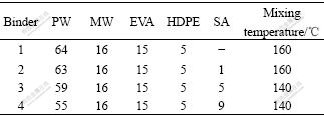
2.2 Processing
Four binders were prepared according to Table 1 and the solid load was 60% (volume fraction). The mixing process was conducted in the torque rheometer (XSS-300) for 2 h at a rotating speed of 40-60 r/min. The mixing temperature was 160 ℃ and 140 ℃ as listed in Table 1. The mixing temperature commonly adopted as Tm+5 ℃ (Tm is the melting temperature of high melting point component). During mixing process, high shear rate made the powder disperse more easily in the binder and eliminated the phenomenon of powder agglomeration effectively.
Granulation was finished in the single-screw extruder (LSJ20). Granulation can mix the feedstock more homogenously in a certain extent and make it convenient to form molded parts in the injection- molding machine.
2.3 Measurement
In current experiment, the viscosity of the feedstock was measured using capillary rheometer (Instron3211). The diameter and the length of the capillary were 0.235 mm and 15.762 mm, respectively. The wetting angles were obtained by the method of dropping melting binder onto the smooth surface of 17-4PH SS board. Fourier infrared-convert spectral tester (NICOLET740, made in America) was used to obtain the FTIR spectra of the SA and the mixture of SA and 17-4PH SS powder. Finally, the specific area of the 17-4PH SS powder was obtained by BET method (measured by the physical adsorption of nitrogen).
3 Results
3.1 Rheologic behavior of feedstock
Viscosity is the most important parameter that judges the rheologic behavior of the MIM feedstock. For example, high viscosity will make it hard to form molded components while low viscosity will make the binder separate from the powder. In this study, the binder used was binder 1, 2, 3 and 4 with a solid loading of 60% (volume fraction). After granulating for three times, viscosity of the feedstock was tested at the temperature of 160 ℃ and the results are listed in Table 2.
Table 2 Viscosity of MIM feedstock (Pa?s)
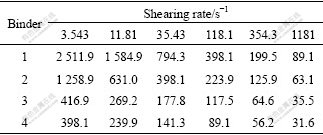
It is acknowledged that viscosity is the internal friction force of running liquid and polymer melt is often considered as pseudo-plastic fluid. At definite temperature, viscosity decreases with the increase of the shear rate that can be expressed as follows:
![]() (1)
(1)
where η is the viscosity, ![]() is the shear rate, K is a coefficient and n is a strain sensitivity coefficient (<1). Fig.2 shows the relationship between the shear rate
is the shear rate, K is a coefficient and n is a strain sensitivity coefficient (<1). Fig.2 shows the relationship between the shear rate ![]() and the viscosity η. In Fig.2, it is clear that viscosity of the feedstock is much higher before adding SA but decreases continuously when adding 1% and 5%SA. While adding more than 5%SA, the viscosity decreases a little and it is quite near in much higher shearing rate when adding 5% and 9%SA. This indicates that the high polymer component plays a leading part in low shearing rate for decreasing viscosity and these components themselves reinforce the wetting property for the powder. Therefore, effects for decreasing viscosity are limited even superfluous surfactants are added. Moreover, the surplus SA will increase the viscosity of the feedstock, which can be explained by cross-linking of polar ends of the surfactant molecules[26].
and the viscosity η. In Fig.2, it is clear that viscosity of the feedstock is much higher before adding SA but decreases continuously when adding 1% and 5%SA. While adding more than 5%SA, the viscosity decreases a little and it is quite near in much higher shearing rate when adding 5% and 9%SA. This indicates that the high polymer component plays a leading part in low shearing rate for decreasing viscosity and these components themselves reinforce the wetting property for the powder. Therefore, effects for decreasing viscosity are limited even superfluous surfactants are added. Moreover, the surplus SA will increase the viscosity of the feedstock, which can be explained by cross-linking of polar ends of the surfactant molecules[26].
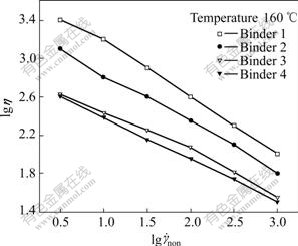
Fig.2 Relationship between shear rate and viscosity
3.2 Wetting angle of binder against 17-4PH SS board
The metal powder (such as 17-4PH SS powder) usually has hydrophilic surface and the organic binder is hard to spread on the powder surface. Thus, the surfactant is required to improve the wetting ability for the powder. Melted binder (the four ingredients in Table 1) was dropped on the surface of 17-4PH SS board (smooth surface) to measure the wetting angle of the binder against the powder. The results are illustrated in Fig.3.
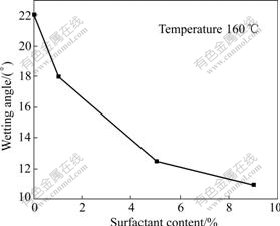
Fig.3 Alteration of wetting angle on 17-4PH SS board
As shown in Fig.3, the wetting angle of the binder against the powder decreases obviously after SA is added. The increase of SA content can decrease the wetting angle in a certain extent. If the content of SA exceeds 5%, its effect for improving the wetting ability is not obvious. It is well known that carboxylic acid molecules in liquid solution can form rather stable dimers[30-32] in particular for those molecules having longer hydrocarbon chains (such as stearic acid). Therefore, the superfluous SA will loss the characteristic of the surfactant for wetting the powder and has no effect for improving the wetting property.
3.3 Critical solid loading of feedstock
Solid loading is the volume fraction of the powder in the feedstock and as generally hoped, the bigger, the better. It is accepted that the maximum solid loading (which is also called critical solid loading), without sacrificing its properties for injection molding, will enhance sintering and minimize shrinkage. The critical solid loading φc has intimate relationships with powder morphology, powder size, particle size distribution, the way of accumulation and the thickness of binder layer on the powder surface.
Table 3 lists the influence of the binder with different ingredients on the critical solid loading. ![]()
![]()
![]() expresses the critical solid loading of binder 1, 2 and 3, respectively. By the way, the critical solid loading was determined by the relationship between the density of the injection-molded compact and the volume fraction of the powder. In Table 3, it is clear that the critical solid loading increases continuously as the SA content increases. The main cause is that the SA content improves the interaction between the powder and the binder. In short, enhancement of the interaction will decrease the usage of the binder and more powder can be contained in the feedstock in a determinate volume. Though the critical solid changes not too much, the increase of the critical solid loading is helpful to improving the sintered density and the performance of the final products. The relationships between the SA content and the critical solid loading will be discussed detailedly in the following section.
expresses the critical solid loading of binder 1, 2 and 3, respectively. By the way, the critical solid loading was determined by the relationship between the density of the injection-molded compact and the volume fraction of the powder. In Table 3, it is clear that the critical solid loading increases continuously as the SA content increases. The main cause is that the SA content improves the interaction between the powder and the binder. In short, enhancement of the interaction will decrease the usage of the binder and more powder can be contained in the feedstock in a determinate volume. Though the critical solid changes not too much, the increase of the critical solid loading is helpful to improving the sintered density and the performance of the final products. The relationships between the SA content and the critical solid loading will be discussed detailedly in the following section.
Table 3 Influence of binder on critical powder loading
![]()
4 Discussion
4.1 Reasons of SA in enhancing dispersibility of feedstock
Binder systems with better dispersibility for different powders are required in the MIM technology. Good dispersibility means that the powder particles will disperse more homogeneously in the binder and the solid loading will become higher under similar viscosity conditions. In current experiment, the SA plays a role of a bridge between the powders and the binder. Therefore, the friction force between the two phases decreases greatly and the powder particles are hard to separate from the binder at high shearing stress. As a result, to decrease the interface power or increase the adsorption force of the two phases is also required in order to get better dispersibility.
The following equation is Yang-Laplace Eqn:
γSL=γSV-γLV cos θ (2)
where γSL is the interfacial tension between the binder and the powder, γSV is the interfacial tension of the powder, γLV is the interfacial tension of the binder and θ is the wetting angle of the liquid binder against the powder.
Additionally, a large number of capillary pores appear among the agglomerated particles before mixing and permeation pressure caused by capillary pores will attract the binder into the pores. The permeation pressure can be expressed as follows:
ΔP=2γLV cos θ/R or ΔP=2(γSV-γSL)/R (3)
where R is the radius of the capillary pipe and γLV is the interfacial tension of the binder.
In Eqn.(2), the decrease of the wetting angle will reduce the interfacial force. Therefore, the binder is prone to spread on the powder surface and the mixing property of the feedstock is improved greatly. In Eqn.(3), the decrease of the wetting angle will increase the permeation pressure. Thus, the binder is easily to be attracted into the pores among the agglomerated particles and this will accelerate the dispersion of the powder in the feedstock system. As a result, the agglomeration phenomenon is eliminated and homogeneity of the feedstock is improved.
The SA can effectively decrease the wetting angle of the binder against the 17-4PH SS powder as shown in Fig.3. Thus, the dispersibility of the feedstock is enhanced according to the discussion above. The primary function of the surfactant is to provide a modification on powder surface so that some degree of steric stabilization between powders can be attained in the given polymer matrix, assisting the powder dispersion in a certain extent.
4.2 Calculations for SA in influencing critical solid loading
For a given powder, if its theoretical density, tap density and apparent density are d0, dt and da, respectively, theoretically, the way of most tightness accumulation is sure to exist in any spherical particles. If the critical bulk density of these particles in this situation is dc, an equation is obvious to exist: d0>dc>dt,
dt=Adc (4)
where A is less than 1 and the value has relationship with powder characteristic. The feedstock is prepared by the following ways: firstly, the binder fills the pores among the particles exactly; secondly, the binder covers the powder and forms a layer on the powder surface with a thickness of H.
In the first step, if the proportion (volume fraction of the binder against the powder) is φb1 and the porosity of the critical accumulation is V, then
![]() (5)
(5)
So
![]() (6)
(6)
Combining formula (4) with formula (5) yields
![]() (7)
(7)
In the second step, powder particles are all supposed to be mono-sized spheres. In a definite volume, the number of the powder particles is n and the surface area of a single powder is πD2 (the powder diameter is D). Then the volume of the binder (the thickness of the surfactant layer is H) on the powder surface is
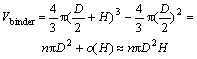 (8)
(8)
where H is much smaller than the size of the powder particles. Therefore, o(H) is the infinite simality of H and can be omitted. In current experiment, the powder surface selected is not smooth and the coarse surface will increase the surface area of the powder in a certain extent. So the adsorptive capacity of the surface shall be revised correspondingly by a parameter B (B>1). As a result, the adsorptive capacity of the powder should be revised as follows:
Vbiner=B×nπD2H=nπBD2H (9)
Then the content of the binder in the layer (compared with the powder) is
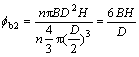 (10)
(10)
It is clear that the critical solid loading φc should be expressed as follows:
![]() (11)
(11)
where
Vbinder/Vpowder=φb1+φb2 (12)
Then,
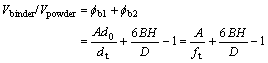 (13)
(13)
Combining Eqn.(11) and Eqn.(13) yields
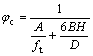 (14)
(14)
For a given powder, A and B are both constants, only φc has relationships with H but H is affected by the interaction between the binder and the powder. Therefore, decreasing the thickness H of the binder layer will increase the solid loading. If the interaction between them is strong, then thin layer is enough to supply better rheologic behavior for the feedstock but also with higher solid loading; otherwise, more binder should be added that will cause the decrease of solid loading. Compared with the three ingredients in Table 3, the improvement of critical solid loading profits from the increase of the SA. The SA helps a lot to strengthen the interaction between the powder and the binder and thus improves the solid loading of the feedstock.
4.3 Reaction principle of SA on 17-4PH SS powder surface
SA is often used as a surfactant in the MIM technology and its molecular formula is C17H35COOH. Its polar group ‘COOH—’ can be adsorbed strongly onto the powder surface and the hydrocarbon chains ‘C17H35—’ spread into the binder. Therefore, the particular structure of the SA solves the compatibility problem between the powder and the organic binder successfully.
Fourier infrared-convert spectral tester was used to obtain the FTIR spectra of SA and the mixture of SA and 17-4PH SS powder, and the results are shown in Fig.4.
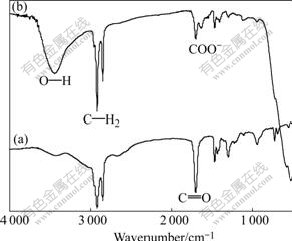
Fig.4 FTIR spectrum for SA (a) and (SA+17-4PH SS powder) mixture (b)
In Fig.4, curve 1 shows the FTIR spectrum of SA. It has a strong band at 1 702 cm-1 due to the C=O group stretch of the carboxyl group. Curve 2 is the FTIR spectrum of the mixture of SA and 17-4PH SS powder. It is distinct that a new band appears at 1 635 cm-1 and another band at 3 447 cm-1 becomes much stronger.
According to FTIR spectra analysis, the first new peak at 1 635 cm-1 is the characteristic of COO- and the second peak at 3 447 cm-1 results from the appearance of water molecules in the mixture. Other researches also get similar FTIR spectra using the mixture of the SA and Al2O3 powder[25].
It is well known that the continuity of the crystal structure is interrupted on the surface and the lattice defects increase because the mass point ordering on the crystal surface decreases. Therefore, the surface structure of the powder is different from the internal structure, which will cause the appearance of unsaturated bond. As a result, the functional groups are prone to be adsorbed on the powder surface and the number of them will increase correspondingly with the increase of the specific area. On the other hand, the effects of functional groups for changing the surface characteristics can not be neglected if a large number of them are adsorbed on the powder surface. Due to the hygroscopicity of the fine powder, water molecules are generally presented on the surface of 17-4PH SS powder as the forms of capillary water and adsorption water. As the deliquescence of the powder surface, O—H is prone to appear on the surface and generates a chemical reaction with the SA. Therefore, a new carboxylate generates after the chemical reaction which can be proved by the band at 1 635 cm-1 in curve 2. Fig.5 illustrates the reaction principle of the chemical reaction. During mixing process, additional water is generated which causes the appearance of the strong band at 3 447 cm-1 in curve 2. This reaction is likely to occur at a proper temperature that was discussed detailedly by NOVAK et al[26] when he studied the surface modification of alumina powder for LPIM. The phenomenon that surfactants generate chemical bonding on the powder surface was also observed by other scholars[33-34]. Moreover, the band at 1 702 cm-1 in curve 2 still exists though it is very weak, indicating that free SA still remains in the mixture due to the incomplete chemical reaction.
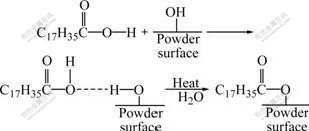
Fig.5 Chemical reaction on surface of 17-4PH SS powder
According to the discussion above, a chemical reaction takes place between SA and SS powder and thus generates a new chemical bonding. Thus, the interaction force between the powder and the binder is strengthened which can explain the effect of the SA in enhancing the solid loading commendably.
4.4 Theoretical estimation for least content of sur- factant
An adsorbing model was used to estimate the least content of the surfactant in the feestock. In this reaction model, the polar groups of the surfactant face, the powder and the oleophilic groups spread into the binder. Fig.6 illustrates the adsorbing model that surfactants generate a monolayer adsorption on the powder surface. In this model, d1 is the diameter of the powder and d2 is the diameter of the polar head of the surfactant. Thus, it is feasible to calculate the content of the surfactant that forms a single molecular layer on the powder surface.
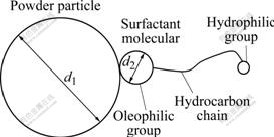
Fig.6 Adsorbing model of surfactant on powder surface
Supposing the powder density is ρp, then the average quality ![]() and surface area of a single powder should be expressed as follows:
and surface area of a single powder should be expressed as follows:
![]() (15)
(15)
![]() (16)
(16)
If the total mass of the powder is WP, the number n of the particles should be
![]() (17)
(17)
Then the total surface area (A) of the powder (its mass is WP) should be
![]() (18)
(18)
For a single surfactant molecule, projected area on the powder surface is about to be
![]() (19)
(19)
For a powder particle, WP is the gross mass and A is the total surface area, the number(N) of the surfactant molecules covered on the powder surface is
![]() (20)
(20)
If NA is Avogadro’s number (6.02×1023) and the mole mass of the surfactant is M, then the mass of the surfactant that forms a single molecular layer on the powder surface is
![]() (21)
(21)
Then, the mass percentage of the surfactant that forms a monolayer adsorption on the powder surface is
![]() (22)
(22)
If φ is the solid loading, WB is the mass of the binder and ρB is the density of the binder, then the mass percentage of the surfactant in the binder is
![]() (23)
(23)
In current experiment, the average particle size of the 17-4 PH SS powder is 12 μm, the binder density is 0.91 g/cm3, the diameter of the polar end of the SA is 5.11×10-10 m[35] and the solid loading is 60% (volume fraction). Then the percentage of the SA to form a single molecular layer on the powder surface is about 0.19%. With the variations of the surfactant, the solid loading and the density of the powder and the binder, the value will have a corresponding change. It is obvious that the surfactant ought to have an optimal content in the binder. According the experimental results above, about 5% is proper for improving the properties of MIM feedstock while the least content of the surfactant needed in theory is only 0.19% that has great difference in value.
Under practical situations, the surface area of the powder depends on the surface morphology. Generally, an equation was used to calculate the specific area of the powder that is expressed as
![]() (24)
(24)
where S is the specific area of the powder and a is a shape coefficient that depends on the particle shapes; ρ and D are the theoretical density and the average particle size of the powder, respectively.
In current investigation, ρ and D are 7.6 g/cm3 and 12 μm, respectively; the shape coefficient a selected is 6 (spheric particles with smooth surface). Then the theoretic value of the specific surface area is about 0.066 m2/g but the practical value is 0.110 m2/g measured by BET method that is a little bigger.
In fact, the shapes of the powder used in the experiment are not all spherical and the surface of them is not smooth. In addition, the powders are not all mono-sized spheres and they have a definite size distribution. More importantly, multilayer adsorption is likely to generate on the powder surface and then the content of the surfactant adsorbed on the powder surface will increase greatly. Therefore, the content of the SA needed in the experiment increased from 0.19% to 5% is reasonable according the discussion above.
5 Conclusions
1) The SA has significant effects for improving the properties of MIM feedstock. It serves as a bridge between the powder and the binder, enhancing the stability of the feedstock. Proper content of the SA can decrease the viscosity of the feedstock and improve the dispersibility of the powder. Moreover, adding of the SA can increase the critical solid loading due to the enhancement of interacting between the powder and the binder.
2) Chemical bonding is found in the mixture of the SA and 17-4 PH SS after mixing, indicating that chemical adsorption occurs on the powder surface with the help of the SA. Thus, the chemical adsorption can reinforce the interacting force between the powder and the binder, enhancing the solid loading of the feedstock system.
3) The content of SA has an optimal value at roughly 5% and the effect for improving the property of the feedstock is limited even excessive SA is added. Indicated by theoretical calculation, 0.19% is enough for the SA to form a single molecule layer on the powder surface but 5% is the proper value in current experiment. The main reasons are likely to be two aspects. First, the powders used are not ideal spheres that with coarse surface and a definite size distribution, increasing the adsorption capacity of the powder. In addition, the adsorption of the SA on the powder surface may be multilayer adsorption, increasing the adsorptive capacity correspondingly.
References
[1] GERMAN R M, HENS K F, LIN S T. Key issues in powder injection molding [J]. Bull Am Ceram Soc, 1991, 70(1): 294-302.
[2] MUTSUDDY B C, FORD R G. Ceramic Injection Molding [M]. London: Chapman & Hall, 1995: 1-22.
[3] GERMAN R M. Technological barriers and opportunities in powder injection molding [J]. Powder Metallurgy, 1993, 25(4): 165-169.
[4] GERMAN R M. Powder Injection Molding [M]. Princeton: Metal Powder Industries Federation, 1990: 12-17.
[5] LOH N H, TOR S B, KHOR K A. Production of metal matrix composite part by powder injection molding [J]. Journal of Materials Processing Technology, 2001, 108(3): 398-407.
[6] MERZ L, RATH S, PIOTTER V, RUPRECHT R, KLEISSL J R, HAUSSELT J. Feedstock development for micro powder injection molding [J]. Microsyst Technol, 2002, 8(1): 29-32.
[7] RUPRECHT R, GIETZELT T, MQLLER K, PIOTTER V, HAUGELT J. Injection molding of microstructured components from plastics [J]. Metals and Ceramics Microsystem Technologies, 2002, 2(8): 351-354.
[8] SUPATI R, LOH N H, KHOR K A, TOR S B. Mixing and characterization of feedstock for powder injection molding [J]. Materials Letters, 2000, 46(2): 109-114.
[9] ZHANG Jian, HUANG Bai-yun, LI Yi-min, LI Song-lin. Influence of microcrystalline wax on properties of MIM multi-component wax matrix binder [J]. Trans Nonferrous Met Soc China, 2002, 12(5): 918-921.
[10] LI Du-xin, QU Xuan-hui, HUANG Bai-yun. Novel PEG based binder system and its debinding properties for MIM [J]. Trans Nonferrous Met Soc China, 2001, 11(1): 90-94.
[11] TARDOS G, KHAN M, MORT P M. Critical parameter and limiting conditions in binder granulation of fine powders [J]. Powder Technology, 1997, 94(3): 246-258.
[12] XIE Zhi-peng, LUO Jie-sheng, WANG Xiu, LI Jian-bao, HUANG Yong. The effect of organic vehicle on the injection molding of ultra-fine zirconia powders [J]. Materials and Design, 2005, 26(1): 79-84.
[13] GERMAN R M, BOSE A. Injection Molding of Metals and Ceramics [M]. Princeton: Metal Powder Industries Federation, 1997: 121-126.
[14] CHAN T Y, LIN S T. Effect of stearic acid on the injection molding of alumina [J]. J Am Ceram Soc, 1995, 78(10): 2746-2752.
[15] MARTYN M T, JAMES R J, HAWORTH B. Injection molding of hard-metal components [J]. Met Powder Rep, 1988, 43: 816-823.
[16] YAN Lu-ting, SI Wen-jie, XIONG Tao, MIAO He-zhuo. The Effects of surfactants on CIM of Si3N4 [J]. Rare Metal Materials and Engineering, 2004, 33(5): 534-538. (in Chinese)
[17] ROMDHANEA M, SAMIR B, JAMEL B, THIERRY CHARTIERB. Dispersion of Al2O3 concentrated suspensions with new molecules able to act as binder [J]. Journal of the European Ceramic Society, 2004, 24(9): 2723-2731.
[18] MOBALLEGH L, MORSHEDIAN J, ESFANDEH M. Copper injection molding using a thermoplastic binder based on paraffin wax [J]. Materials Letters, 2005, 59: 2832-2837.
[19] WRIGHT J K, EDIRISINGHE M J, ZHANG J G. Particle packing in ceramic injection molding [J]. J Am Ceramic Soc, 1990, 73(9): 2653-2658.
[20] PUGH R J, BERGSTROM L. Surface and Colloid Chemistry in Advanced Ceramics Processing [M]. New York: Marcel Dekker, 1994: 127-130.
[21] WENJEA J T, TENG K H. The effect of surfactant adsorption on sedimentation behaviors of Al2O3 toluence suspensions [J]. Mater Sci Eng, 2001, 318(1): 102-110.
[22] WENJEA J T, LIU D M, HSU C K. Influence of stearic acid on suspension structure and green microstructure of injection-molded zirconia ceramics [J]. Ceramics International, 1999, 25(5): 191-195.
[23] JOHNSON R E, MORRISON W H. Ceramic powder dispersion in non-aqueous system [J]. Adv Ceram, 1987, 21: 323-327.
[24] DOROSZKOWSKI A, LAMBOURNE R. Faraday discussions of the chemical society [J]. Chem Soc, 1978, 65: 252-263.
[25] LIN S T, GERMAN R M. Interaction between binder and powder in injection molding of alumina [J]. J Mater Sci, 1994, 29(5): 207-212.
[26] NOVAK S, VIDOVIC K, SAJKO M, KOSMAC T. Surface modification of alumina powder for LPIM [J]. Journal of the European Ceramic Society, 1997, 17(2): 217-223.
[27] LI Song-lin, HUANG Bai-yun, QU Xuan-hui, LI Yi-min. Influence of surfactants on properties of metal injection molding (MIM) feedstock [J]. Rare Metal Materials and Engineering, 2001, 30(2): 131-134.
[28] QUACKENBUSH C L, FRENCH K, NIEL J T. Fabrication of sinterable silicon nitride by injection molding [J]. Ceram Eng Sci Proc, 1982, 3: 20-34.
[29] BAYER A G .Thermoplastic molding compounds [P]. USA 6774166, 1994-08-10.
[30] SMALL D M. The Physical Chemistry of Lipids [M]. New York: Plenum, 1986: 31-35.
[31] SEIFERT G, PATZLAFF T, CRAENER H. Picosecond vibtation energy transfer observed in CH and OH stretching region of stearic acid dimers in liquid solution [J]. Journal of Molecular Liquids, 2003, 102(1): 227-240.
[32] KRAACK H, DEUTSCH M, OCKO B M, PERSHAN P S. The structure of organic langmuir films on liquid metal surfaces [J]. Nuclear Instruments and Methods in Physics Research , 2003, 200: 363-370.
[33] HENDRIK A. CAPELLE, BRITCHER L G, MORRIS G E. Sodium stearate adsorption onto titania pigment [J]. Journal of Colloid and Interface Science, 2003, 268(2): 293-300.
[34] NIANQIANG W, LEI F, MING S, MOHAMMED A, KA C W, VINAYAK P D. Interaction of fatty acid monolayers with cobalt nanoparticles [J]. Nano Letters, 2004, 4(2): 383-386.
[35] ZHU Yao, ZHAO Zhen-guo. Interface Chemistry [M]. Beijing: Chemical Industry Press, 1996: 42-50.
___________________________________
Foundation item: Project(200135) supported by Chinese National Excellent Dissertation Foundation; Project(NECT-04-0754) supported by Program for New Century Excellent Talents in University of China
Corresponding author: LI Yi-min; Tel: +86-731-8830693; E-mail: liyimm@yahoo.com.cn

