J. Cent. South Univ. Technol. (2011) 18: 381-385
DOI: 10.1007/s11771-011-0707-5
Effect of L-cysteine on bioleaching of Ni-Cu sulphide by A. manzaensis
HE Zhi-guo(贺治国)1, 2, ZHAO Jian-cun(赵健存)1, 2, LIANG Wan-jie(梁万洁)1, 2,
HU Yue-hua(胡岳华)1, 2, QIU Guan-zhou(邱冠周)1, 2
1. School of Minerals Processing and Bioengineering, Central South University, Changsha 410083, China;
2. Key Laboratory of Biometallurgy of Ministry of Education, Central South University, Changsha 410083, China
? Central South University Press and Springer-Verlag Berlin Heidelberg 2011
Abstract: The effect of L-cysteine in different concentrations on the bioleaching of Ni-Cu sulfide was studied with an extremely thermophilic archaea, Acidianus manzaensis. It is found that adding certain amounts of L-cysteine to the bioleaching system of Ni-Cu sulfide largely enhances the leaching rate. X-ray diffraction (XRD) patterns show the change of bioleached solid residues and the effect of L-cysteine on the surface charges of minerals. Zeta potential and IR spectra of mineral surface show that the interaction between L-cysteine and mineral leads to the formation of metal complex, which is propitious to the bioleaching of Ni-Cu sulfide by Acidianus manzaensis.
Key words: Acidianus manzaensis; L-cysteine; Ni-Cu sulfide; bioleaching; surface charge; Zeta potential
1 Introduction
Attempts to improve the metal recovery include the enhancement of bacterial activity and the modification of the area of exposed sulfides by surface active agents or bio-macromolecules. Recent studies have shown that the recovery of metals from sulfide ores can be improved when carbohydrates, proteins and other substances of biological origin are added into the leaching media for bacteria [15]. For example, the dissolution of metallic ions from alloys is increased by the addition of amino acids and/or albumin [17]. These procedures must be done in economical and environmentally safe manner.
L-cysteine, as an important sulfureous amino acid, has been widely used in medicine, food, cosmetic and feeding stuff industry [7]. Recently, L-cysteine is focused in the field of microbial metallurgy [8, 12] as well due to its capacity to accelerate the leaching rate [12, 16]. It was reported that the active group sulfydryl (—SH) of the molecule was involved in bio-leaching process [3, 13-15, 19]. Previous reports showed that the recovery of metals can be improved by Acidithiobacillus ferrooxidans [3,15] and Acidithiobacillus caldus [2] when L-cysteine is added to the leaching system of sulfide.
Acidianus manzaensis (A. manzaensis) is a novel thermoacidophilic archaeon which could grow autotrophically using H2 or S0 as an electron donor andFe3+ as an electron acceptor, and also could grow heterotrophically using some organic compounds. Fe3+ and O2 served as electron acceptors for growth. However, S0, NO3, NO2, 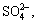 Mn4+, fumarate and Fe2O3 did not serve as electron acceptors. The ranges of growth temperature and pH for A. manzaensis were 60-90 °C (optimum of 80 °C) and pH 1.0-5.0 (optimum pH of 1.2-1.5), respectively [9]. Cells were nearly regular cocci with an envelope comprised of the cytoplasmic membrane and a single outer S-layer. A. manzaensis plays an important role in biooxidation processes associated with biomining. For example, it is used to treat chalcopyrite and pyrite. As one of the most effective sulfide oxidizers recorded, A. manzaensis has been suggested to be included in the removal of inhibitory sulfur from mineral surfaces and the production of surfactants.
Mn4+, fumarate and Fe2O3 did not serve as electron acceptors. The ranges of growth temperature and pH for A. manzaensis were 60-90 °C (optimum of 80 °C) and pH 1.0-5.0 (optimum pH of 1.2-1.5), respectively [9]. Cells were nearly regular cocci with an envelope comprised of the cytoplasmic membrane and a single outer S-layer. A. manzaensis plays an important role in biooxidation processes associated with biomining. For example, it is used to treat chalcopyrite and pyrite. As one of the most effective sulfide oxidizers recorded, A. manzaensis has been suggested to be included in the removal of inhibitory sulfur from mineral surfaces and the production of surfactants.
In this work, the effects of L-cysteine on the bioleaching of Ni-Cu sulfide by A. manzaensis were studied by XRD, Zeta potential determination and IR spectra observation to further understand the roles of organic substances in bioleaching.
2 Experimental
2.1 Bacterial strains and media
The pure culture of A. manzaensis YN25 used in this study was isolated and identified on the basis of its physiological characteristics and chromosomal DNA base composition. And it was maintained in medium 9K with the initial pH value of 2.5 in a liquid medium containing 1% (mass fraction/volume fraction) sulfur and 0.2 g/L yeast extract at 65 °C. The composition of medium 9K is as follows: (NH4)2SO4 3 g/L, KCl 0.1 g/L, K2HPO4 0.5 g/L, MgSO4·7H2O 0.5 g/L, Ca(NO3)2 0.01 g/L and FeSO4·7H2O 44.7 g/L.
2.2 Preparation of minerals sample
The Ni-Cu sulfide was prepared by grinding and sieving to a particle diameter of 70-100 μm (over 85%), which contains 35.04% S, 1.32% Cu, 56.7% total iron and 3.76% Ni. Its chemical composition is given in Table 1.
Table 1 Chemical compositions of Ni-Cu sulfide samples (mass fraction, %)

2.3 Experimental procedure
The extremely thermophilic archaea was grown in liquid 9K media containing 2% (mass fraction/volume fraction) dry mineral and the heat-sterilized solution that was adjusted to pH 1.5 with sulfuric acid. The initial density of cells was approximately 1×107 mL-1. To determine the contribution of acid leaching, the sterile cell-free control cultures were also used in each set of experiments. All experiments were carried out with 250 mL flasks containing 100 mL of solution. Flasks were placed into a HZ-9613Y constant temperature vibrator (200 r/min) and incubated at 65 °C.
2.4 Analysis methods
Suitable amount of solution was sampled from each flask every two days. The pH value, oxidation-reduction potential and the cell concentration in solution were measured. The pH value of the bioleaching system was measured with a pH probe calibrated with a low pH buffer. The oxidation-reduction potential was detected by platinum electrode. The cell concentration in solution was determined by direct counting using hemocytometer under an optical microscope. The content levels of copper and nickel in solution were analyzed by atomic absorption spectrophotometry. Zeta potential determination and IR spectra observations were adopted to show the effect of L-cysteine on the surface charge of minerals, and the mechanism of interaction with the mineral surface. The surface features of solid residues and mineral composition changes were analyzed by X-ray diffractometry (XRD).
3 Result and discussion
3.1 Effect of L-cysteine on metal sulphide bioleaching
It can be seen in Fig.1 that the concentrations of Cu2+ in the leaching solutions are increased with leaching time except for the cell-free control. Certain amounts of L-cysteine can significantly improve the bioleaching of Ni-Cu sulfide. The copper is leached at a higher rate than that without L-cysteine added for all L-cysteine concentrations used except for 0.4 g/L. The leaching rate of copper reaches the highest when 0.2 g/L of L-cysteine is added. Then, it is considered that the optimal concentration of L-cysteine should be 0.2 g/L in this study.
The leaching of Ni from the Ni-Cu sulfide by A. manzaensis is shown in Fig.2. From Fig.2, it can be seen that different amounts of Ni are leached when different amounts of L-cysteine are added. The concentration of dissolved Ni reaches the highest when 0.2 g/L L-cysteine is added. A lower leaching rate of Ni is obtained as 0.4 g/L L-cysteine is added, indicating that excessive L-cysteine is poisonous to bioleaching Ni from Ni-Cu sulfide by A. manzaensis.

Fig.1 Concentrations of dissolved copper during Ni-Cu sulfide bioleaching (Incubation concentration: 107 mL-1; Amount of Ni- Cu sulfide applied: 20 g/L)
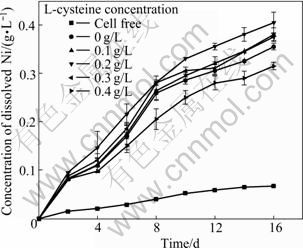
Fig.2 Concentrations of dissolved nickel during Ni-Cu sulfide bioleaching (Incubation concentration: 107 mL-1; Amount of Ni-Cu sulfide applied: 20 g/L)
Fig.3 shows the relationship between the growth of bacteria and the initial L-cysteine concentration. The growth of cells is slightly inhibited by L-cysteine at a concentration of 0.3 g/L or 0.4 g/L, while L-cysteine at concentrations of 0.1 and 0.2 g/L can improve the bacterial growth. This indicates that appropriate amount of L-cysteine can promote the growth of A. manzaensis in the bioleaching system of Ni-Cu sulfide, while excessive L-cysteine can cause the opposite effect.
Fig.4 indicates the effect of different concentrations of L-cysteine on the pH values. Only in the presence of A. manzaensis, the pH value of the bioleaching system of Ni-Cu sulfide increases from the initial 1.5 to 2.2 in the first two days and subsequently decreases. At last, the pH value is around 1.3. However, under the action of L-cysteine, the pH value of the bioleaching systems of Ni-Cu sulfide reaches around 1.7 at the fourth day and maintains about 1.2 in the end. It can be seen that L-cysteine can slow down the increase of the pH value of the bioleaching system. Low pH value is propitious to the growth of A. manzaensis and the bioleaching of Ni-Cu sulfide.
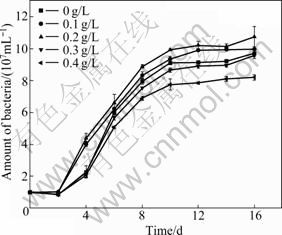
Fig.3 Cell concentrations during Ni-Cu sulfide bioleaching at different concentrations of L-cysteine (Incubation concentration: 107 mL-1; Amount of Ni-Cu sulfide applied: 20 g/L)
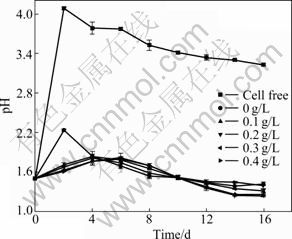
Fig.4 Change of pH during Ni-Cu sulfide bioleaching at different concentrations of L-cysteine (Incubation concentration: 107 mL-1; Amount of Ni-Cu sulfide applied: 20 g/L)
The L-cysteine remarkably enhances the oxidation- reduction potential of the Ni-Cu sulfide bioleaching system, as shown in Fig.5. Without L-cysteine, the oxidation-reduction potential of the Ni-Cu sulfide bioleaching system reaches around 575 mV in the presence of A. manzaensis. However, the oxidation- reduction potential achieves about 610 finally with L-cysteine. The oxidation-reduction potential of the bioleaching system has a certain relationship with the ratio of ferric to ferrous ion concentrations. The higher oxidation-reduction potential means that more ferrous ions are oxidized to ferric ions, which is in favor of the Ni-Cu sulfide bioleaching. Therefore, the appropriate amount of L-cysteine can promote the bioleaching of the Ni-Cu sulfide by raising the oxidation-reduction potential of the bioleaching system.
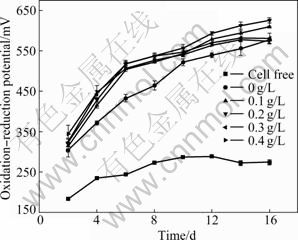
Fig.5 Oxidation-reduction potential during Ni-Cu sulfide bioleaching at different concentrations of L-cysteine (Incubation concentration: 107 mL-1; Amount of Ni-Cu sulfide applied: 20 g/L)
3.2 Effect of L-cysteine on surface charge of minerals
The surface charge of Ni-Cu sulfide treated with different concentrations of L-cysteine is shown in Fig.6. When the concentration of L-cysteine is lower than 3 g/L, the Zeta potentials of Ni-Cu sulfide move positively with the increase of L-cysteine concentration, which may be due to the fact that the mercapto [—SH] of L-cysteine adsorbed on the surface of the sulfide and amino group increases the Zeta potential of minerals [4, 16]. When the concentration of L-cysteine is higher than 3 g/L, Zeta potentials of Ni-Cu sulfide reduces with the increase of L-cysteine concentration. This shows that the L-cysteine absorbed on Ni-Cu sulfide is saturated when the concentration of L-cysteine is 3 g/L.
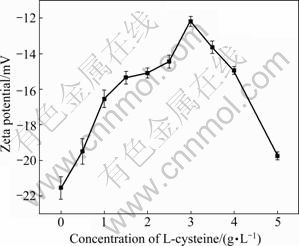
Fig.6 Effect of L-cysteine concentration on Zeta potential of Ni-Cu sulfide
3.3 XRD analysis
XRD patterns of bioleached Ni-Cu sulfide indicate its chemical compositions. According to the XRD analysis [10], the Ni-Cu sulfide is mainly composed of pyrrhotite (Fe1-xS and Fe7S8), chalcopyrite (CuFeS2) and S. It can also be seen that compared with the cell-free Ni-Cu sulfide scoria, the intensities of some XRD peaks of Ni-Cu sulfide scoria bioleached by A. manzaensis with different concentrations of L-cysteine added are changed obviously. The bioleached Ni-Cu scoria includes jarosite, ammoniojarosite, pyrrhotite and pentlandite. It is found that the peak densities of sulfur decrease and some of the peaks of sulfur disappear for the bioleached Ni-Cu scoria with different concentrations of L-cysteine added, which is due to the ability of bacteria to oxidize elemental sulfur to 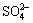 [1,18]. This is consistent with the previous reports for the bioleaching of pyrrhotite [4, 11].
[1,18]. This is consistent with the previous reports for the bioleaching of pyrrhotite [4, 11].
3.4 IR spectra analysis of interaction between L- cysteine and Ni-Cu sulfide
The IR absorption spectra of Ni-Cu sulfide before and after treated with L-cysteine are shown in Fig.7. There are distinct differences in the range of wavelength from 3 100-500 cm-1 on the IR spectrum of Ni-Cu sulfide incubated with 0.2 g/L L-cysteine. L-cysteine has amido, carboxyl and —HS groups. It has been reported in Refs.[6, 21] that, the amino group is located at 3 300- 3 200 cm-1 (S—NH2), and 2 962.86 cm-1(N—H) where a broad adsorption peak appears; —HS turns up at 2 551.02 cm-1. Several broad and scattered adsorption peaks of carboxylic acid appear at 1 423.78 cm-1 (COO-), 1 296.17 cm-1 (C—O) and 1 062.64 cm-1 (O—H). From Fig.7, after L-cysteine is added, the peak intensity of Ni-Cu sulfide at 614 cm-1 and 1 130 cm-1 increases. At the same time, the five new peaks appear, at 846, 1 410, 1 580, 2 090 and 3 020 cm-1, respectively. These all suggest that L-cysteine has adsorbed on Ni-Cu sulfide surfaces. The bands at 1 580 and 3 020 cm-1 characterize asymmetric —NH2 stretching and bending, respectively. The bands at 1 410 cm-1 is due to carboxylic integrating with Ni-Cu sulfide. The bands near 846 and 2 090 cm-1 are the characteristic peaks of Fe-cysteine and —C=O group. These suggest that L-cysteine absorbs on Ni-Cu sulfide surfaces through amino, carboxyl and —HS groups.

Fig.7 FTIR spectra before (a) and after (b) interaction between L-cysteine and Ni-Cu sulfide
The previous report shows that the thiol species interacting with the sulfide will play a dominant role in the disruption of the interface and the transport of the sulfur species to the bacterial cell. L-cysteine contains —HS group which is a reduced sulfur source or a substrate for the organisms. L-cysteine addition provides an additional reduced sulfur source for the bacteria which competes with CuS-NiS and FeS. Normally, organisms would oxidize the reduced sulfur in L-cysteine to produce H2SO4, reducing the pH. The reduction in the pH and the availability of another substrate (L-cysteine) should decrease the bioleaching extent of mineral sulfide. However, biomass concentration would be increased in the presence of L-cysteine due to the utilization of L-cysteine. L-cysteine could interact with the mineral sulfides by forming hydrogen-bonds on the surface, increasing the Zeta potential of the mineral surfaces and hence improving the absorption of negatively charged bacteria to the mineral sulfide surfaces. The roles of L-cysteine in bioleaching are assumed that the interaction disrupts the structure and leads to the formation of a metal ion-cysteine complex, a cysteine-mineral complex (bonded via a sulfur bridge) and an interfacial SH group [16]. These complexes are the putative chemical energy carriers for organisms with a cyclic turnover of cysteine and metal ion. Therefore, the interaction and the overall effect of L-cysteine are complicated.
4 Conclusions
1) The suitable amount of L-cysteine can improve the biological activity of A. manzaensis, and greatly increase the bioleaching rate of Ni-Cu sulfide. In order to achieve the largest leaching rate of metal, the optimal concentration of L-cysteine should be 0.2 g/L with a fixed mass fraction of Ni-Cu sulfide (2%).
2) It is found that L-cysteine adsorbs on Ni-Cu sulfide surfaces through amino, carboxyl and —HS groups, which leads to the change of the Ni-Cu sulfide structure and the formation of a metal-cysteine complex. L-cysteine increases the hydrophilicity on the minerals surface, which is beneficial for the bacteria to contact with the minerals.
3) The adsorbed amount is saturated when L-cysteine concentration is over 3 g/L with a fixed mass fraction of Ni-Cu sulfide (2%) shown by Zeta potential.
References
[1] FU Jian-hua, QIU Guan-zhou, LIU Jian-she, HU Yue-hua, ZHANG Zai-hai. Study on bacterial leaching of oxide-sulphide copper ores [J]. Mining and Metallurgical Engineering, 2003, 23(4): 30-34.
[2] HE Zhi-guo, GAO Feng-ling, ZHONG Hui, HU Yue-hua. Effects of L-cysteine on Ni-Cu sulfide and marmatite bioleaching by Acidithiobacillus caldus [J]. Bioresource Technology, 2009, 100(3): 1383-1387.
[3] HU Yue-hua, HE Zhi-guo, HU Wei-xin, PENG Hong, ZHONG Hui. Effect of two kinds of amino-acids on bioleaching metal sulfide [J]. Transactions of Nonferrous Metals Society of China, 2004, 14(4): 794-797.
[4] LI Hong-mei, KE Jia-jun. The progress in bioleaching of nickel-bearing sulfide ores [J]. Multipurpose Utilization of Mineral Resources, 1999, 5(3): 28-33.
[5] LIU Jian-she, WANG Zhao-hui, LI Bang-mei, ZHANG Yan-hua. Interaction between pyrite and cysteine [J]. Transactions of Nonferrous Metals Society of China, 2006, 16(4): 943-946.
[6] LIU Jing-ping, LI Jin, GE Xing. Synthesis and free radical inhibition rate of copper(II), iron(II), manganese(II) complexes with cysteine [J]. Chemical World, 2004(5): 235-238. (in Chinese)
[7] LIU Zhong, YANG Wen-bo, BAI Gang, TIAN Wang, JIN Yon-jie. Microbial enzyme conversion of L-cysteine and L-cystine [J]. Microbiology, 2003, 30(6): 16-21. (In Chinese)
[8] MIN Xiao-bo, CHAI Li-yuan, CHEN Wei-liang, ZHANG Chuan-fu, HUANG Bai-yun, KUANG Zhong. Study on bioleaching of refractory gold ore (I)—Mechanism on bioleaching of pyrite by Thiobacillus ferroxidans [J]. Transactions of Nonferrous Metals Society of China, 2001, 11(5): 784-789.
[9] YOSHIDA N, NAKASATO M, OHMURA N, ANDO A, SAIKI H, ISHII M, IGARASHI Y. Acidianus manzaensis sp. nov., a novel thermoacidophilic archaeon growing autotrophically by the oxidation of H2 with the reduction of Fe3+ [J]. Current Microbiology, 2006, 53(5): 406-411.
[10] PARKER A, KLAUBER C, KOUGIANOS A, WATLING H R, VAN BRONSWIJK W. An X-ray photoelectron spectroscopy study of the mechanism of oxidative dissolution of chalcopyrite [J]. Hydrometallurgy, 2003, 71(1/2): 265-276.
[11] QIU Guan-zhou, LI Quan, QIN Wen-qing. Bacterial leaching complex sulfide minerals of lead, antimony, zinc and iron [J]. Mining and Metallurgical Engineering, 2005, 25(3): 30-33.
[12] QIU Guan-zhou, LIU Jian-she, HU Yue-hua. Electrochemical behavior of chalcopyrite in presence of Thiobacillus ferroxidans [J]. Transactions of Nonferrous Metals Society of China, 2000, 10(S1): 23-25.
[13] ROJAS-CHAPANA J A, BARTELS C C, POHLMANN I, TRIBUTSCH H. Co-operative leaching and chemotaxis of thiobacilli studied with spherical sulphumulphide substrates [J]. Process Biochem, 1998, 33(3): 239-248.
[14] ROJAS-CHAPANA J A, GIERSIG M, TRIBUTSCH H. The path of sulphur during the bio-oxidation of pyrite by Thiobacillus ferrooxidans [J]. Fuel, 1996, 75(8): 923-930.
[15] ROJAS-CHAPANA J A, TRIBUTSCH H. Biochemistry of sulfur extraction in bio-corrosion of pyrite by Thiobacillus ferrooxidans [J]. Hydrometallurgy, 2001, 59(2/3): 291-300.
[16] ROJAS-CHAPANA J A, TRIBUTSCH H. Bio-leaching of pyrite accelerated by cysteine [J]. Process Biochemistry, 2000, 35(8): 815-824.
[17] SANO Y, TAKEDA S. Cytotoxicity and dissolution of metallic biomaterials using dynamic extraction (in vitro) [J]. Shika Igaku, 1992, 55(2): 125-140.
[18] SUZUKI I. Microbial leaching of metals from sulphide minerals [J]. Biotechnology Advances, 2001, 19(2): 119-132.
[19] TRIBUTSCH H, BENNET J C. Semiconductor-electrochemical aspects of bacterial leaching: I. Oxidation of metal sulphides with large energy gaps [J]. J Chem Techno1 Biotechnol, 1981, 31(1): 565-511.
[20] TRIBUTSCH H, ROJAS-CHAPANA J A. Metal sulfide semiconductor with the reduction of Fe3+ [J]. Current Microbiology, 2006, 53(3): 406-411.
[21] YUE Song. Preparations and structure characterization of the crystals of ferrous cysteine [J]. Chemical Research and Application, 2000, 12(4): 387-390. (in Chinese)
(Edited by YANG Bing)
Foundation item: Projects(50621063, 30400010) supported by the National Natural Science Foundation of China; Project(2010CB630903) supported by the National Basic Research Program of China
Received date: 2010-03-26; Accepted date: 2010-06-02
Corresponding author: HU Yue-hua, Professor, PhD; Tel: +86-731-88879815; E-mail: hyh@mail.csu.edu.cn