采用锰矿从水溶液中吸附铅和镉离子
来源期刊:中国有色金属学报(英文版)2012年第12期
论文作者:Aylin SÖNMEZAY M. SALIM ÖNCEL Nihal BEKTAŞ
文章页码:3131 - 3139
关键词:铅;镉;锰矿;吸附动力学;等温吸附;金属吸附
Key words:lead; cadmium; manganoxide minerals; adsorption kinetics; adsorption isotherm; metal adsorption
摘 要:采用低成本的本地可得的天然锰矿作为吸附剂,研究吸附工艺从水溶液中脱除铅离子和镉离子。利用伪一级、伪二级动力学和颗粒内扩散模型检验动力学吸附数据,计算和比较这些动力学模型的吸附速率常数,发现用伪二级动力学模型能最佳地描述吸附动力学。将Langmuir 和 Freundlich 等温吸附模型用来拟合不同温度下的平衡数据,发现实验数据与Langmuir 模型拟合得更好。采用Langmuir 等温吸附模型计算出锰矿吸附铅离子和镉离子的最大容量分别为98 和 6.8 mg/g。计算了热力学参数,如吸附吉布斯自由能的变化、焓变与熵变。结果表明,锰矿作为吸附剂对铅和镉的吸附反应是自发的吸热反应。因此,锰矿作为一种天然的矿物吸附剂,可以替代现有的吸附剂来脱除水溶液中的铅离子和镉离子。
Abstract: Removal of lead and cadmium ions from aqueous solutions by adsorption process was investigated. Low cost and locally available natural mineral of manganoxide mineral was used as an adsorbent. The kinetics of adsorption process data was examined using the pseudo-first-order, pseudo-second-order kinetics and the intra-particle diffusion models. The rate constants of adsorption for all these kinetics models were calculated and compared. The adsorption kinetics was best described by the pseudo second-order model. The Langmuir and Freundlich adsorption isotherm models were applied to the experimental equilibrium data at different temperatures. The experimental data well fitted to Langmuir isotherm model. The maximum adsorption capacities of manganoxide mineral for lead and cadmium ions were calculated from the Langmuir isotherm and were 98 and 6.8 mg/g, respectively. Thermodynamic parameters such as the change of Gibbs free energy, enthalpy and entropy of adsorption were also calculated and it was found that the lead and cadmium uptake reactions by manganoxide mineral were endothermic and spontaneous in nature. Therefore, manganoxide mineral can be used as adsorbents for lead and cadmium ions removal processes as an alternative natural mineral among the others.
Trans. Nonferrous Met. Soc. China 22(2012) 3131-3139
Aylin  , M. SALIM
, M. SALIM  , Nihal
, Nihal 
Gebze Institute of Technology, Department of Environmental Engineering, 41400 Gebze, Turkey
Received 2 February 2012; accepted 7 May 2012
Abstract: Removal of lead and cadmium ions from aqueous solutions by adsorption process was investigated. Low cost and locally available natural mineral of manganoxide mineral was used as an adsorbent. The kinetics of adsorption process data was examined using the pseudo-first-order, pseudo-second-order kinetics and the intra-particle diffusion models. The rate constants of adsorption for all these kinetics models were calculated and compared. The adsorption kinetics was best described by the pseudo second-order model. The Langmuir and Freundlich adsorption isotherm models were applied to the experimental equilibrium data at different temperatures. The experimental data well fitted to Langmuir isotherm model. The maximum adsorption capacities of manganoxide mineral for lead and cadmium ions were calculated from the Langmuir isotherm and were 98 and 6.8 mg/g, respectively. Thermodynamic parameters such as the change of Gibbs free energy, enthalpy and entropy of adsorption were also calculated and it was found that the lead and cadmium uptake reactions by manganoxide mineral were endothermic and spontaneous in nature. Therefore, manganoxide mineral can be used as adsorbents for lead and cadmium ions removal processes as an alternative natural mineral among the others.
Key words: lead; cadmium; manganoxide minerals; adsorption kinetics; adsorption isotherm; metal adsorption
1 Introduction
Heavy metals present in environments can cause severe damage to human and aquatic life. Accumulation of these metals in the environment elements such as in food chain may pose a significant danger to human health. Lead and cadmium can be present in wastewater from metallurgical alloying, electroplating, photography, pigmenting, textile printing, chemical industries batteries and lead mine drainage [1,2]. Governments and environmental agencies set limit values (Table 1) in terms of the allowable concentration for lead and cadmium in drinking water and wastewaters [3-7]. Therefore, the removal of these ions from wastewaters has become a very important manner. Several methods for removal of metal ions from wastewaters have been proposed, but most of them have disadvantages such as precipitation and coagulation and become less effective and more expensive in situations involving high volumes and low metal concentrations. In addition, these methods can also create sludge disposal problem. Membrane processes are also limited in their use due to high capital investment and operational costs. Such processes for effluent treatment are well established and discussed in Refs. [8-11].
The adsorption process with low cost natural minerals is an attractive option because of their efficient removal rate for heavy metal ions at even trace levels [12,13]. Well-designed adsorption processes provide high quality effluent after treatment. In recent years, considerable attention has been focused on the removal of heavy metals from aqueous solutions by using adsorbents derived from low-cost materials, especially natural minerals [14-16].
Insoluble metal hydroxides such as manganoxides can participate in a wide range of redox reactions with both organic and inorganic compounds. This allows forming an electrostatic binding between pollutants and the minerals. Therefore, these minerals can be classified as a potential sustainable and cost effective adsorbent for metal removal from aqueous solution. Manganoxide minerals contain manganese and oxygen or the hydroxyl ion as principal elements and also comprise oxides, carbonates and silicates. The manganese oxide minerals are basically formed in octahedron structural units forming a manganese ion surrounded by six oxygen atoms (MnO6). This structure is connected to another either corners with one oxygen atom or edges with two oxygen atoms. They can be formed into several oxide minerals depending on the surface conditions of nature. All these valances form a large number of manganoxide minerals, namely, pyrolusite, psilomelane, manganite, rodokrosite, cryptomelane, hollandite and coronadite. Most of manganese minerals are black and opaque, and the hardness of 5-7. They are rarely crystalline in structure and occur in unequal stacks or grains [17,18]. Manganese minerals can be found as a small deposit at many locations in Turkey.
Therefore, the aim of this work is to explore lead and cadmium adsorption characteristics of manganoxide mineral. This possible use of natural clay manganoxide minerals as an adsorbent material for metal ions removal from aqueous solutions was presented.
Table 1 Tolerance and discharge limits for lead and cadmium in drinking water and surface water


Fig. 1 XRD pattern and SEM images of manganoxide minerals
2 Material and methods
2.1 Chemicals and characterization of manganoxide minerals
All chemicals were analytical grade and used without further treatment. Deionized water was used in all experiments. Pb(NO3)2 and CdCl2·1/2H2O were used to prepare stock metal solutions.
Manganoxide minerals obtained from Burdur region, Turkey were used in this work. The mineral sample was received as a bloc. Therefore, the samples were grounded and sieved to obtain different particle sizes and used throughout the experiments. The main physical and chemical properties of the minerals samples were evaluated by XRD (Rigaku D-max 1000), XRF (EDX-600B Skyray) and SEM (Philips XL30 SFEG).
The XRD analyses were carried out to two different parts of the manganoxide mineral bloc. XRD results indicate that softer side of sample bloc is mainly pyrolusite (Fig. 1(a)). Broad amorphous peaks were obtained from XRD pattern (not given here) for the harder part mineral sample. This part was named as psilomelane since it has poor crystalline structure and marking black from physical observation. This manganoxide bloc (the mixture of psilomelane and pyrolusite) was crushed and then uniformly mixed and used throughout in the experiments. Chemical composition of the mineral sample was also determined by XRF analysis shown in Table 2. The SEM image of manganese oxide were taken to determine the surface morphology and presented in Fig. 1(b). The image in the smallest magnification, ellipse-like shapes of adsorbent particles can be seen easily. Cave and pores on the surface of the adsorbent where metal ions can pass through can be seen in the image with a higher magnification. The images also show that the surface structure of manganoxide particle is highly heterogeneous.
2.2 Kinetic and isotherm experiments
Batch experiments were conducted to investigate the kinetics and equilibrium data in 100 mL of glass flasks as it is essential for designing and operation of adsorption process for wastewater treatment. A known amount of adsorbent (1 g) was weighed and placed in flask containing 50 mL solution at desired concentrations which were 250 and 50 mg/L for lead and cadmium, respectively [19]. Separate flasks were used for each time interval and one flask was taken and analyzed for desired time. Three sets of isotherm plots were obtained at three different temperatures (25, 35, 45 °C). Each isotherm consisted of eight adsorbate concentrations varied from 25 to 1000 mg/L and 25 to 2500 mg/L for cadmium and lead ions, respectively.
Table 2 Chemical composition of manganoxide mineral used in the experiments (mass fraction, %)

Final metal concentrations were measured in the equilibrium solution after separating the manganoxide mineral through the filtration paper. Inductively coupled plasma, ICP (Perkin Elmer, Optima 7000DV) was used to measure the concentrations of lead and cadmium ions in the solutions. The concentration retained in the adsorbent phase was calculated using Eq. (1). In order to investigate the mechanism of adsorption, three different models were used as the kinetic parameters gave important information for designing and modelling the process. These models’ linear Eqs. (2)-(4) are as follows:
 (1)
(1)
where qt is the amount of metal ions adsorbed at time t, mg/g; ρ0 is the initial metal ion concentration, mg/L; ρt is metal ion concentration, mg/L; V is the volume of solution, L; Ws is the mass of the adsorbent, g.
 (2)
(2)
where qe is the amount of arsenic ion on the surface of the adsorbent at the equilibrium, mg/g; k1 is the equilibrium rate constant of pseudo-first sorption, min-1; t is the time, min.
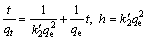 (3)
(3)
where  is the equilibrium rate constant of pseudo- second order, g/(mg·min); h is the initial sorption rate, mg/(g·min).
is the equilibrium rate constant of pseudo- second order, g/(mg·min); h is the initial sorption rate, mg/(g·min).
 (4)
(4)
where qt is amount of adsorbate on the surface of the adsorbent at time t, mg/g; ki is the intro-particle rate constant, mg/(g·min1/2).
The equilibrium relationships between adsorbent and adsorbate are described by adsorption isotherms, the ratio between the amount adsorbed and that remaining in the solution at a fix temperature is equilibrium. In this work, metal removal process was analyzed using Langmuir and Freundlich isotherm models (Eqs. (5) and (6)).
 (5)
(5)
where Q0 (mg/g) and b (L/mg) are Langmuir isotherm constants. The value of Q0 gives the maximum adsorption capacity of adsorbent.
 (6)
(6)
where KF and n are Freundlich isotherm constants that can be related to the adsorption capacity and the adsorption intensity, respectively.
3 Results and discussion
3.1 Effect of contact time and adsorbent dose
Lead and cadmium ion removal rates against contact time at initial concentrations of 250 and 50 mg/L, respectively, are presented in Fig. 2. It was clearly seen that the adsorption of the lead ions increased instantly at the initial stage and reached the equilibrium. However, the adsorption of the cadmium ions increased instantly at the initial stage and continued to increase gradually until the equilibrium was reached and remained constant. The equilibrium time was selected to be 120 min for both lead and cadmium ions. The maximum amounts of lead and cadmium ions adsorbed on the manganoxide mineral were 11.5 and 2.2 mg/g, respectively.

Fig. 2 Plot of lead (250 mg/L) (a) and cadmium (50 mg/L) (b) ions removal rates against contact time (300 r/min, 1 g, 25 °C)
Figure 3 shows the experimental results obtained from series of experiments using different adsorbent doses, ranging from 0.05 to 0.3 g. Adsorbent dosage is an important parameter because it determines the capacity of an adsorbent for a given initial concentration of the adsorbate. As shown in Fig. 3, the uptake of metal ions increased with increasing the adsorbent dose until 1 g for both ions. Therefore, 1 g manganoxide was used throughout the experiments.
Figure 4 shows that the adsorption of lead and cadmium slightly increased with decreasing the particle size. As the adsorption being a surface phenomenon, the smaller sorbent sizes offered comparatively larger surface areas and hence higher removal at equilibrium. For further experiments, the size of 1.18-0.425 mm was selected because of its high removal capacities as well as easy handling.

Fig. 3 Effect of adsorbent dose on removal rate

Fig. 4 Effect of adsorbent particle size on removal rate
3.2 Adsorption kinetics
In order to predict the adsorption kinetic models of metal ions, the pseudo-first order and pseudo-second order kinetics models (Eqs. (2)-(4)) were applied to experimental data. For the pseudo-first adsorption rate constant, the straight line plots of lg (q1-qt) against time were calculated [20,21]. The equilibrium rate constant of pseudo-second order was calculated by plotting t/qt against t [22]. The kinetics constants and correlation coefficients of all kinetics models were calculated and given in Table 3. Good correlation coefficients were obtained for the pseudo second-order kinetics model, which showed that both metal uptake processes followed the pseudo-second order rate expression.
Adsorption kinetics is usually controlled by different mechanisms and the intra-particle diffusion model is generally used to identify the mechanism involved in the process. Considering a solid–liquid adsorption process, it is also common that external mass transfer is dominant at the beginning of adsorption and then gradually the adsorption is controlled by intra-particle diffusion as the external surface of adsorbent loaded with the adsorbate. According to the model, proposed by WEBER and MORRIS [23], the initial rate of intra-particle diffusion can be calculated by plotting q against t1/2. The equation for intra-particle diffusion model is given as Eq. (4).
Only intra-particle plots for lead and cadmium ions with manganoxide minerals are given in Fig. 5. Two parts of linear portions of figures indicated that the lead and cadmium ions were initially adsorbed by the exterior surface of the adsorbent. The saturation was reached and metal ions entered into pores within the particles and were adsorbed by the interior surfaces. The diffusion rate parameters for two sections of intra-particle plot were obtained from the plots and given in Table 3. The correlation coefficients for the intra-particle diffusion model varied from 0.89 to 0.98. However, any of plots passed through the origin. Therefore, it can be concluded that intra-particle diffusion was involved in the adsorption process but it was not the rate-controlling step. The data indicated that external mass transfer (surface adsorption) was only significant at the beginning of the process. Simultaneously, intra-particle diffusion controlled the adsorption at the final stage (Fig. 5). Similar type of adsorption kinetics was reported for lead ion adsorption onto modified and unmodified kaolinite clay [24] and also lead and cadmium adsorption by biosorption [25]. The intercept value provided information about the boundary layer thickness, namely, the larger the intercept is, the greater the boundary layer effect. The slope of the linear portion showed the rate of the adsorption. The lower slope, seen in the second part of the linear curve, corresponds to a slower adsorption process.
Table 3 Kinetic constants and correlation coefficients of kinetic models used


Fig. 5 Intra particle diffusion model
3.3 Adsorption isotherms
The equilibrium experiments were conducted at three different initial temperatures (25, 35, 45 °C). Adsorption isotherms were obtained by experimental data (Fig. 6) using Eqs. (5)-(6). The isotherm constants were calculated and presented in Table 4. These two isotherm models were validated by each linearised plot. As shown in Table 4, the correlation coefficients (R2) obtained from the Langmuir isotherm model (R2 >0.998) were higher than the Freundlich model for both metals. Therefore the Langmuir equation better represents the adsorption processes. It can be said that all sites on the adsorbent possess equal affinity for the adsorbate.
The separation parameter values of RL, were calculated using Eq. (7). The RL values for the lead and cadmium adsorption process were found in the range of 0.019-0.450 and 0.0010-0.102, respectively. These values indicated (0
 (7)
(7)
where RL is dimensionless separation parameter.
The maximum adsorption capacities (Q) for lead and cadmium ions were calculated to be 9.8 and 6.8 mg/g from the Langmuir plots, respectively. The increase in adsorption capacity of adsorbent at higher temperatures may be due to the enlargement of pore size or activation of the adsorbent surface. The maximum adsorption capacities of cryptomelane type manganoxide mineral were reported to be 60 and 9.8 mg/g of lead and cadmium, respectively, by FENG et al [26]. The maximum lead and cadmium removal capacities of calcitic limestone material were 40 and 1.3 mg/g [27]. The monolayer adsorption capacity of Turkish illitic clay was reported to be 13.09 and 53.7 mg/g for cadmium and lead ions, respectively [28]. Therefore, it can be said that the these values can be compared with the other similar data reported in the literature. These results also showed that adsorption capacity of cadmium ion was lower than that of lead ions. The decrease can be attributed to the ionic properties such as ion radius, charge, ionic potential, electronegativity, and the electron configuration of each metal ion. Electronegativity is a chemical property that describes the ability of an atom to attract electrons towards itself in a covalent bond. Therefore, the surface complexation reaction is more influenced by the electrostatic attraction between the surface charge and the dissolved ions. The electronegativivity of Pb2+ (2.33) is slightly higher than that of Cd2+ (1.69), so lead ions interact more strongly electrostatically with the surface groups present on the surface of the adsorbent.

Fig. 6 Equilibrium isotherm plots for lead (a) and cadmium (b) ions
Table 4 Isotherm constants and correlation coefficients

On the other hand, the tendency to lose water molecules from the aquo-cations is stronger for Pb2+ since the single ion hydration enthalpy is -1481 kJ/mol for Pb+2 and -1807 kJ/mol for Cd2+. This also facilitates the interaction between Pb2+ and the adsorbent surface. Another factor, which may determine the metal adsorption capacity, is the ionic radius itself. The smaller ions with the same valency, have higher charge densities and attract more water molecules, resulting in a higher hydrated radius. As a result, Pb2+ has a higher ionic radius (1.21  ) and subsequently a smaller hydrated radius than Ca2+ causing weaker interaction between Ca2+ and adsorbent. It can be therefore said that lead ions can penetrate into smaller pores and, thus, have a large access to the adsorbent surface.
) and subsequently a smaller hydrated radius than Ca2+ causing weaker interaction between Ca2+ and adsorbent. It can be therefore said that lead ions can penetrate into smaller pores and, thus, have a large access to the adsorbent surface.
3.4 Thermodynamic evaluation of process
Thermodynamic parameters, i.e., free energy of adsorption (△GΘ), standard enthalpy (△HΘ) and entropy changes (△SΘ) were evaluated at different temperatures (25-45 °C) using Eqs. 8-10. △HΘ and △SΘ were obtained from the slop and intercept of a plot of ln Kd against 1/T (Fig. 7). Langmuir constant b (L/mg) was converted to adsorption equilibrium constant (Kd) by multiplying molar mass of adsorbate [29,30]. LUI [29,30] reported that for neutral adsorbates or adsorbates with weak charge, the Langmuir equilibrium constant can be reasonably used for the thermodynamic equilibrium constant of adsorption (Kd). As a result, the use of the Langmuir equilibrium constant for calculation of ΔGΘ and subsequent determination of ΔHΘ and ΔSΘ is numerically correct.
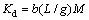 (8)
(8)
where Kd is the equilibrium constant; b is the Langmuir isotherm constant; M is relative molecular mass.
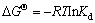 (9)
(9)
where △GΘ is free energy of sorption, kJ/mol.
 (10)
(10)
The thermodynamic parameters were calculated and given in Table 5. The Gibbs free energy specifies the degree of spontaneity of adsorption process. The negative △GΘ values indicate that the adsorption was spontaneous. Increasing temperature does not significantly change △GΘ for both ions showing reaction rate not much affected with temperature. The positive values of △HΘ indicate that the adsorption process is endothermic in nature. The positive entropy change (△SΘ) shows an increased randomness at the adsorbent– adsorbate interface during the adsorption process. Similar observation was reported for the adsorption of lead and cadmium ions on illite [28].

Fig. 7 Plots of ln Kd against 1/T for lead (a) and cadmium (b) ions
Table 5 Thermodynamic parameters

4 Conclusions
1) Low cost manganoxide minerals used in this work is an effective adsorbent for removing lead and cadmium ions from aqueous solution. The adsorption kinetics and equilibrium of the removal process were determined in a batch operation mode.
2) Kinetic evaluation shows that the pseudo-second order kinetic reaction model is found to represent the data better for each metal removal process. The intra-particle diffusion model shows that external mass transfer is significant at the initial adsorption stage and then the intra-particle diffusion controls the adsorption rate at the final stage.
3) The isotherm data correlated better by the Langmuir isotherm model and the maximum adsorption capacities are 98 and 6.8 mg/g, respectively. Thermo-dynamical parameters were also evaluated and show that the adsorption of the metal ion is endothermic in nature. The results show that locally available and inexpensive manganoxide minerals can be useful adsorbents for removing metal ions from aqueous solution.
References
[1] FAWELL J K,  G. Pollution; causes, effects and control, 3rd edition [J].
G. Pollution; causes, effects and control, 3rd edition [J].  R M. The Royal Society of Chemistry, London, 1996: 59.
R M. The Royal Society of Chemistry, London, 1996: 59.
[2] AGARWAL S K. Heavy metal pollution [J]. New Delhi: APH Publishing, 2009: 39.
[3] WHO. Guidelines for Drinking-Water Quality, 2nd edition, Geneva, 1993.
[4] European Union Council Directive for water quality [S]. The drinking water directive (DWD), Council Directive 98/83/EC, http://ec.europa.eu/environment/water/water-drink/index_en.html, 2012.
[5] EPA. National Primary Drinking Water Regulations, http://water.epa.gov/drink/contaminants/index.cfm#Inorganic, 2012.
[6] Republic of Turkey, Ministry of Health, Turkish Regulation on Drinking Water Intended for Human Consumption, No: 25730, Ankara, Turkey, 2005.
[7] Republic of Turkey, Ministry of Environment and Forestry, Turkish Water Pollution Control Regulation, No: 25687, Ankara, Turkey, 2004.
[8]  F, WANG Q. Removal of heavy metal ions from wastewaters: A review [J]. Journal of Environmental Management, 2011, 92(3): 407-418.
F, WANG Q. Removal of heavy metal ions from wastewaters: A review [J]. Journal of Environmental Management, 2011, 92(3): 407-418.
[9] WAN NGAH W S, HANAFIAH MAKM. Removal of heavy metal ions from wastewater by chemically modified plant wastes as adsorbents: A review [J]. Bioresource Technology, 2008, 99(10): 3935-3948.
[10] XIONG C H, YAO C. Adsorption behavior of gel-type weak acid resin (110-H) for Pb2+, Transactions of Nonferrous Metals Society of China, 2008 18(5): 1290-1294.
[11] ORTEGA L M, LEBRUN R, BLAIS J F, HAUSLER R. Removal of metal ions from an acidic leachate solution by nanofiltration membranes [J]. Desalination, 2008, 227(1-3): 204-216.
[12] BABEL S, KURNIAWAN T A. Low-cost adsorbents for heavy metals uptake from contaminated water: A review [J]. Journal of Hazardous Materials, 2003, 97(1-3): 219-243.
[13] YANG R T. Adsorbents: Fundamentals and applications [J]. New Jersey: John Wiley & Sons, Inc, 2003: 1.
[14] GUPTA S S, BHATTACHARYYA K G. Kinetics of adsorption of metal ions on inorganic materials: A review [J]. Advances in Colloid and Interface Science, 2011, 162(1-2): 39-58.
[15] BHATTACHARYYA K G, GUPTA S S. Adsorption of a few heavy metals on natural and modified kaolinite and montmorillonite: A review [J]. Advances in Colloid and Interface Science, 2008, 140(2): 114-131.
[16] AHMARUZZAMAN M. Industrial wastes as low-cost potential adsorbents for the treatment of wastewater laden with heavy metals [J]. Advances in Colloid and Interface Science, 2011, 166(1-2): 36-59.
[17] CHETTERJEE K K. Use of metal and metallic minerals [J]. New Age International, New Delhi, 2007: 111.
[18] CORATHERS L A, MACHAMER J F, MANGANESE IN KOGEL J E, TRIVEDI N C, BARKER J M, KRUKOWSKI S T. (Ed), Industrial Minerals and Rocks. CO: SME, Colorado, 1994, 631.
[19]  A. Removal of cadmium and lead ions from aqueous solutions by adsorption using manganoksit mineral as an adsorbent [D]. Turkey: Gebze Institute of Technology, 2011. (in Turkish)
A. Removal of cadmium and lead ions from aqueous solutions by adsorption using manganoksit mineral as an adsorbent [D]. Turkey: Gebze Institute of Technology, 2011. (in Turkish)
[20] LAGERGREN S. Zur theorie der sogenannten adsorption  stoffe [J]. Kungliga Svenska Vetenskapsakademiens. Handlingar, 1898, 24: 1-39.
stoffe [J]. Kungliga Svenska Vetenskapsakademiens. Handlingar, 1898, 24: 1-39.
[21] HO Y S. Citation review of Lagergren kinetic rate equation on adsorption reactions [J]. Scientometrics, 2004, 59: 171-177.
[22] HO Y S, MCKAY G. Pseudo-second order model for sorption processes [J]. Process Biochemistry, 1999, 34: 451-465.
[23] WEBER W J, MORRIS J C. Kinetics of adsorption on carbon from solution [J]. Journal of the Sanitary Engineering Division, 1963, 89(2): 31-60.
[24] UNUABONAH EI, ADEBOWALE KO, OLU-OWOLABI B I. Kinetic and thermodynamic studies of the adsorption of lead (II) ions onto phosphate-modified kaolinite clay [J]. Journal of Hazardous Materials, 2007, 144: 386-395.
[25] ARECO M, DOS SANTOS AFONSO M. Copper, zinc, cadmium and lead biosorption by Gymnogongrus torulosus [J]. Thermodynamics and kinetics studies. Colloids and Surfaces B: Biointerfaces, 2010, 81: 620-628.
[26] FENG X H, ZHAI L M, TAN W F, LIU F, HE J Z. Adsorption and redox reactions of heavy metals on synthesized Mn oxide minerals [J]. Environmental Pollution, 2007, 147: 366-373.
[27] 
 M P. Lead and cadmium immobilization on calcitic limestone materials [J]. Desalination, 2010, 262(1-3): 1-10.
M P. Lead and cadmium immobilization on calcitic limestone materials [J]. Desalination, 2010, 262(1-3): 1-10.
[28] OZDES D, DURAN C, SENTURK H B. Adsorptive removal of Cd(II) and Pb(II) ions from aqueous solutions by using Turkish illitic clay [J].Journal of Environmental Management, 2011, 92(12): 3082-3090.
[29] LIU Y. Some consideration on the Langmuir isotherm equation [J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2006, 274: 34-36.
[30] LIU Y. Is the free energy change of adsorption correctly calculated [J]. Journal of Chemical & Engineering Data, 2009, 54: 1981-1985.
Aylin  , M. SALIM
, M. SALIM  , Nihal
, Nihal 
Gebze Institute of Technology, Department of Environmental Engineering, 41400 Gebze, Turkey
摘 要:采用低成本的本地可得的天然锰矿作为吸附剂,研究吸附工艺从水溶液中脱除铅离子和镉离子。利用伪一级、伪二级动力学和颗粒内扩散模型检验动力学吸附数据,计算和比较这些动力学模型的吸附速率常数,发现用伪二级动力学模型能最佳地描述吸附动力学。将Langmuir 和 Freundlich 等温吸附模型用来拟合不同温度下的平衡数据,发现实验数据与Langmuir 模型拟合得更好。采用Langmuir 等温吸附模型计算出锰矿吸附铅离子和镉离子的最大容量分别为98 和 6.8 mg/g。计算了热力学参数,如吸附吉布斯自由能的变化、焓变与熵变。结果表明,锰矿作为吸附剂对铅和镉的吸附反应是自发的吸热反应。因此,锰矿作为一种天然的矿物吸附剂,可以替代现有的吸附剂来脱除水溶液中的铅离子和镉离子。
关键词:铅;镉;锰矿;吸附动力学;等温吸附;金属吸附
(Edited by LI Xiang-qun)
Corresponding author: M. SALIM  ; Tel: +90-262-6053208; Fax: +90-262-6053205; E-mail: soncel@gyte.edu.tr
; Tel: +90-262-6053208; Fax: +90-262-6053205; E-mail: soncel@gyte.edu.tr
DOI: 10.1016/S1003-6326(12)61765-8

