Formation mechanism of MgB2 in 2LiBH4 + MgH2 system for reversible hydrogen storage
来源期刊:中国有色金属学报(英文版)2011年第5期
论文作者:寇化秦 肖学章 陈立新 李寿权 王启东
文章页码:1040 - 1046
关键词:配位氢化物;LiBH4;MgB2;储氢;形成机理
Key words:complex hydride; LiBH4; MgB2; hydrogen storage; formation mechanism
摘 要:对2LiBH4+MgH2体系放氢过程中MgB2的形成条件及机理进行研究。结果表明:在较高的4.0×105 Pa初始氢背压下放氢时,会抑制2LiBH4+MgH2体系中LiBH4的自行分解,进而使其与MgH2分解放氢后生成的Mg发生反应生成MgB2,同时在450 °C、9.6 h内释放出9.16%(质量分数)的氢气;而在较低的1.0×102 Pa初始氢背压下放氢时,体系中LiBH4会先行发生自行分解,从而不能与Mg发生反应生成MgB2,在450 °C、5.2 h内只能放出7.91%的氢气。2LiBH4+MgH2体系放氢生成MgB2可以使放氢反应进行得更彻底,并释放出更多的氢气。2LiBH4+MgH2放氢时MgB2的形成过程是一个孕育?长大的过程,随着氢背压的增高,孕育期缩短;而随着反应温度的降低,孕育期延长。
Abstract:
The formation conditions of MgB2 in 2LiBH4 + MgH2 system during dehydrogenation were investigated and its mechanism was discussed. The results show that direct decomposition of LiBH4 is suppressed under relative higher initial dehydrogenation pressure of 4.0×105 Pa, wherein LiBH4 reacts with Mg to yield MgB2, and 9.16% (mass fraction) hydrogen is released within 9.6 h at 450 °C. However, under relatively lower initial dehydrogenation pressure of 1.0×102 Pa, LiBH4 decomposes independently instead of reacting with Mg, resulting in no formation of MgB2, and 7.91% hydrogen is desorbed within 5.2 h at 450 °C. It is found that the dehydrogenation of 2LiBH4 + MgH2 system proceeds more completely and more hydrogen desorption amount can be obtained within a definite time by forming MgB2. Furthermore, it is proposed that the formation process of MgB2 includes incubation period and nucleus growth process. Experimental results show that the formation process of MgB2, especially the incubation period, is promoted by increasing initial dehydrogenation pressure at constant temperature, and the incubation period is also influenced greatly by dehydrogenation temperature.

KOU Hua-qin, XIAO Xue-zhang, CHEN Li-xin, LI Shou-quan, WANG Qi-dong
Department of Materials Science and Engineering, Zhejiang University, Hangzhou 310027, China
Received 16 May 2010; accepted 29 November 2010
Abstract: The formation conditions of MgB2 in 2LiBH4 + MgH2 system during dehydrogenation were investigated and its mechanism was discussed. The results show that direct decomposition of LiBH4 is suppressed under relative higher initial dehydrogenation pressure of 4.0×105 Pa, wherein LiBH4 reacts with Mg to yield MgB2, and 9.16% (mass fraction) hydrogen is released within 9.6 h at 450 °C. However, under relatively lower initial dehydrogenation pressure of 1.0×102 Pa, LiBH4 decomposes independently instead of reacting with Mg, resulting in no formation of MgB2, and 7.91% hydrogen is desorbed within 5.2 h at 450 °C. It is found that the dehydrogenation of 2LiBH4 + MgH2 system proceeds more completely and more hydrogen desorption amount can be obtained within a definite time by forming MgB2. Furthermore, it is proposed that the formation process of MgB2 includes incubation period and nucleus growth process. Experimental results show that the formation process of MgB2, especially the incubation period, is promoted by increasing initial dehydrogenation pressure at constant temperature, and the incubation period is also influenced greatly by dehydrogenation temperature.
Key words: complex hydride; LiBH4; MgB2; hydrogen storage; formation mechanism
1 Introduction
Hydrogen is an ideal secondary energy carrier for application because of its highly energy content and environmental harmony. However, efficient hydrogen storage and transportation is a key technical challenge in promoting its further applications. LiBH4, which owns high gravimetric and volumetric hydrogen densities (18.5% in mass fraction and 121 kg/m3), has been regarded as one of the most promising hydrogen storage materials[1]. The hydrogen desorption reaction at elevated temperature (>400 °C) proceeds as follows[2]:
LiBH4→LiH+B+3/2H2 (1)
Upon the overall dehydriding reaction, 13.5% of hydrogen can be released from LiBH4. Unfortunately, it is experimentally shown that LiBH4 is thermodynamically stable for practical use, and it is required for extremely rigorous reaction conditions to reverse[3-4]. VAJO et al[5-6] developed a hydrogen storage system composed of LiBH4 and MgH2, in which LiBH4 was effectively destabilized and reaction reversibility was improved. The reversible dehydrogenation/rehydrogenation reaction is expressed as follows:
2LiBH4+MgH2?MgB2+2LiH+4H2 (2)
Interestingly, previous experiments show that a hydrogen atmosphere is necessary for dehydrogenation according to reaction (2). If under dynamic vacuum, however, MgB2 is not formed, and dehydrogenation follows another reaction path expressed as[7]
2LiBH4+MgH2→Mg+2LiH+2B+4H2 (3)
Meanwhile, calorimetric measurements by NAKAGAWA et al[8] showed the formation of MgB2 unless under H2 atmosphere rather than inert gas. PRICE et al[9-11] found that the dehydrogenation pathway was hardly affected by the stoichiometry ratio of LiBH4 to MgH2 and Li-Mg alloy was formed under dynamic vacuum after dehydrogenation at high temperature. PINKERTON et al[7] reported a thermodynamically and kinetically boundaries of the H2 pressure-temperature for formation of MgB2 in TiCl3-catalyzed 2LiBH4+ MgH2 system. A wide sloping plateau was observed by B?SENBERG et al[12] during dehydrogenation of neat 2LiBH4+MgH2 system. However, there is still no further investigation on appearance reason of the plateau. Although the impact of hydrogen atmosphere on the appearance of MgB2 in the product has been studied extensively, little investigation on full understand of the formation of MgB2 has been done as yet.
Because MgB2 plays an important role in reversible hydrogen storage, it is significant to reveal the detailed connection between comprehensive reaction conditions and formation of MgB2. Therefore, in the present work, we investigated the intrinsic formation mechanism of MgB2 during the dehydrogenation of 2LiBH4+MgH2 system.
2 Experimental
LiBH4 (95% in mass fraction) and MgH2 (98% in mass fraction) were purchased from Alfa Aesar Corp. All materials were used as-received in powder form. The sample of 2LiBH4 + MgH2 system was mechanically milled under 1 MPa hydrogen pressure in a Planetary mill at 400 r/min for 2 h. The milling vessel and balls were made of stainless steel. The ball to powder mass ratio was in around 40:1. All sample operations were performed in a glovebox under continuous purified argon atmosphere. Hydrogen desorption behaviors of the samples were monitored with a Sievert’s type apparatus. Dehydriding performance started from a finite initial hydrogen pressure with heating to aimed temperature at a constant ramping rate of 5 °C/min. Each time, 150-250 mg sample was put into a closed large reaction sample volume (820 mL), which resulted in (0.2-0.3)×105 Pa pressure change after dehydrogenation. The identification of the samples was carried out by X-ray diffractometry (XRD, X'Pert-PRO, Cu Kα radiation). To prevent H2O and O2 contamination during the measurements, a special sample holder was used.
3 Results and discussion
3.1 Investigation on dehydrogenation process of 2LiBH4+MgH2 system
The dehydriding behaviors of 2LiBH4 + MgH2 system were measured firstly by heating to 450 °C and under initial dehydrogenation pressure of 1.0×102 Pa and 4.0×105 Pa, respectively, as shown in Fig.1. Because the pressure increase during the dehydrogenation is small, the initial dehydrogenation pressure almost presents the reaction pressure circumstance. It can be seen that the dehydriding curve under 1.0×102 Pa initial hydrogen gas back-pressure exhibits a two-step feature, whereas roughly three-step feature under 4.0×105 Pa initial dehydrogenation pressure. For both samples, their first dehydrogenation steps are similar, after which about 2.5% hydrogen is released. Evident difference appears after the first dehydrogenation step. Under 1.0×102 Pa initial dehydrogenation pressure, hydrogen is released acutely as temperature increases, reaching 7.91% within 5.2 h at 450 °C. In comparison, the dehydriding curve under 4.0×105 Pa initial dehydrogenation pressure exhibits a sloping plateau with slow hydrogen desorption. However, about 6 h later, hydrogen then evolves rapidly, and hydrogen desorption capacity of 9.16% is finally obtained within 9.6 h.
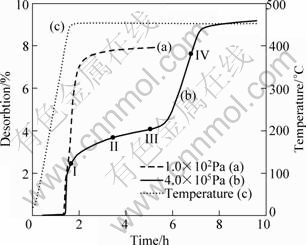
Fig.1 Dehydriding curves of 2LiBH4+MgH2 systems performed under initial dehydrogenation pressure of 1.0×102 Pa (a) and 4.0×105 Pa (b) and temperature profile (c) at ramping rate of 5 °C/min
Figure 2 shows the XRD patterns of the dehydrogenated samples performed under 1.0×102 Pa and 4.0×105 Pa initial dehydrogenation pressure, respectively. When an initial dehydrogenation pressure of 4.0×105 Pa is applied, MgB2, Mg and LiH are produced (Fig.2(b)). On the other hand, when an initial hydrogen pressure of 1.0×102 Pa is applied, three phases of Mg, B and LiH are produced (Fig.2(a)). Unfortunately, no diffraction peak of boron can be observed, suggesting that boron is amorphous, which agreed with other references[2-3]. These results confirm the impact of initial dehydrogenation pressure on dehydriding products of LiBH4-MgH2 system that the formation of MgB2 needs hydrogen overpressure of a fraction of MPa, and relatively lower initial H2 gas back-pressure leads to Mg and B produced instead of MgB2. A little MgO phase may be caused by oxidation during loading. The broad peak around 2θ=15° corresponds to the thin film of sample holder.
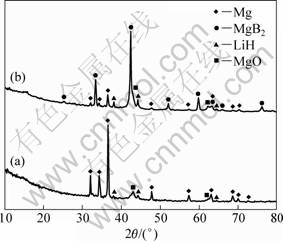
Fig.2 XRD patterns of dehydrogenated 2LiBH4+MgH2 systems performed under initial dehydrogenation pressures of 1.0×102 Pa (a) and 4.0×105 Pa (b)
To understand the formation process of MgB2 and the effectiveness of the plateau in the dehydriding curve under 4.0×105 Pa initial dehydrogenation pressure, we performed XRD phase analysis at the different dehydriding stages (I, II, III and IV points in Fig.1(b)) in the dehydrogenation process, as shown in Fig.3. Figure 3(a) shows the XRD pattern of 2LiBH4+MgH2 mixture prepared by mechanical milling. After dehydrogenation proceeding for 1.7 h, the XRD pattern in Fig.3(b) corresponds to LiBH4 and Mg metal, indicating that MgH2 has decomposed into Mg and H2 relative to the first dehydrogenation step, described as reaction (4). For XRD pattern in Fig.3(c) corresponding to dehydriding for 3.5 h, there is a little LiH besides LiBH4 and Mg, but no MgB2 appears, which can be seen from the illustrated pattern. This indicates that a small amount of LiBH4 has decomposed. Then, nearly no changes display in Fig.3(d) after dehydriding for 5 h, which is in accordance with the appearance of plateau in Fig.1(b). However, strong peaks of MgB2 arise when the reaction duration extends to 6.8 h, as shown in Fig.3(e). Meanwhile, some LiBH4 and Mg are detected still. After the overall dehydrogenation completed about 9.6 h later, massive MgB2 has formed and a small quantity of Mg is obtained, and no LiBH4 can be observed simultaneously (Fig.2(b)). This suggests that MgB2 is formed from the reaction of LiBH4 and Mg, which can be described as reaction (5). Combined with the dehydriding curve of Fig.1(b), the dehydriding process of 2LiBH4 + MgH2 system under 4.0×105 Pa initial dehydrogenation pressure can be described as follows: along with temperature increasing to 450 °C, MgH2 is firstly decomposed, forming Mg and releasing H2; when temperature holding at 450 °C, a small amount of LiBH4 is slowly decomposed; over 6 h extending, MgB2 is uninterruptedly produced accompanying with a large amount of H2 evolved. Thus, the dehydrogenation process of 2LiBH4+MgH2 at 450 °C under 4.0×105 Pa initial dehydrogenation pressure can be summarized as three steps: 1) decomposition of MgH2 to form Mg and H2; 2) decomposition of a small amount of LiBH4; 3) fast dehydrogenation of LiBH4 to react with Mg and form MgB2. Obviously, steps 2) and 3) present two dehydrogenation pathways of LiBH4-MgH2 system under relatively higher initial dehydrogenation pressure and relatively lower initial dehydrogenation pressure, respectively.
MgH2→Mg+H2 (4)
Mg+2LiBH4→MgB2+2LiH+3H2 (5)
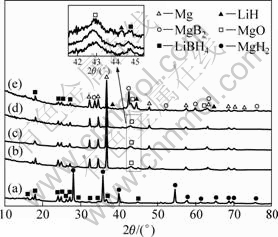
Fig.3 XRD patterns of 2LiBH4+MgH2 systems at different dehydriding stages performed under 4.0×105 Pa initial dehydrogenation pressure before dehydrogenation (a) and after dehydriding for 1.7 h (b), 3.5 h (c), 5 h (d) and 6.8 h (e) (Peak identifications of LiBH4 are originated from Ref.[3].)
It can be found that dehydrogenation pathway under higher initial pressure (recorded as DP(1)) is the sequence of reaction (4) and reaction (5). However, dehydrogenation pathway under lower initial pressure (recorded as DP(2)) is nothing but the result of physical stacking of reaction (4) and reaction (1). The key difference between DP(1) and DP(2) originates from the second dehydrogenation reaction. Under relatively higher initial dehydrogenation pressure, direct decomposition of LiBH4 is suppressed and LiBH4 can react with Mg to yield MgB2. Conversely, LiBH4 decomposes independently under relatively lower initial dehydrogenation pressure. So, the dehydrogenation pathway is greatly dependence of reaction hydrogen pressure.
3.2 Investigation on influence of initial dehydro- genation pressure
Figure 4 shows dehydriding behaviors of 2LiBH4 + MgH2 system applied to different initial hydrogen pressures from room temperature to 450 °C. The dehydriding curves under 1.0×105 Pa and 2.0×105 Pa initial hydrogen pressure demonstrate a two-step dehydrogenation. For both of them, the first step is similar, with desorbed hydrogen of about 2.5%. However, the second hydrogen desorption step under 2.0×105 Pa initial hydrogen pressure is slower than that under 1.0×105 Pa. After holding at 450 °C for 13 h, desorption of 8.05% and 8.12% hydrogen are released under 1.0×105 Pa and 2.0×105 Pa initial hydrogen pressure, respectively. Three-step dehydrogenation is obviously observed under 3.0×105 Pa and 4.0×105 Pa together with 4.8×105 Pa initial hydrogen pressure, in all which dehydriding plateau and fast releasing of hydrogen after the plateau can be identified. Furthermore, it can be found that the hydrogen desorption rate of the second step corresponding to decomposition of LiBH4 and dehydriding plateau become slower and shorter with increasing initial pressure. These plateaus consume approximately are 7.5, 4 and 3 h for initial hydrogen pressure of 3.0×105, 4.0×105, 4.8×105 Pa, respectively. Simultaneously, the hydrogen desorption rate in the third step increases gradually with increasing initial hydrogen pressure. As a result, 9.1% and 9.0% hydrogen have been released within 9 h under 4.0×105 Pa and 4.8×105 Pa initial hydrogen pressure, while 7.9% hydrogen is released within even 13 h under 3.0×105 Pa initial hydrogen pressure. It can be concluded that more complete dehydrogenation of 2LiBH4+MgH2 occurs and more hydrogen desorption amount can be obtained within a definite time with increasing initial dehydrogenation pressure.

Fig.4 Dehydriding curves of 2LiBH4 + MgH2 systems performed under different initial dehydrogenation pressures and temperature profile at temperature ramping rate of 5 °C/min
The XRD patterns of the dehydrogenated 2LiBH4+MgH2 samples applied to different initial hydrogen pressures are shown in Fig.5. All dehydriding products of different initial hydrogen pressures consist of Mg, MgB2 and LiH. Nevertheless, the peak intensity of Mg appears much weaker along with increasing hydrogen pressure, while the peak intensity of MgB2 appears much stronger. Compared the products of reaction (2) with reaction (3), it is found that LiH exists in both reactions. Simultaneously, it is noteworthy that boron produced in reaction (3) cannot be characterized by XRD[2-3]. Therefore, the dehydrated product, Mg or MgB2, is the signal of each dehydrogenation pathway. MgB2 represents DP(1), while the presence of Mg metal in the products represents DP(2). Due to the LiBH4 to Mg molar ratio of 2:1, the relative content of MgB2 to Mg in the products implies that the relative proportion of LiBH4 that reacts with Mg or decomposes independently. In other words, the relative diffraction intensity of MgB2 to Mg metal in the XRD patterns implies the occurrence rate of DP(1) or DP(2) in the overall dehydrogenation. If only MgB2 phase exists in the products, it means that the whole dehydrogenation reaction proceeds as DP(1). The dehydrogenation reaction entirely follows DP(2), in contrast, only when Mg metal phase exists in the products. According to Fig. 5, we can conclude that both DP(1) and DP(2) appear in the whole dehydrogenation reaction under various initial hydrogen pressures. However, with increasing the initial dehydrogenation pressure, dehydrogenation prefers to follow DP(1).
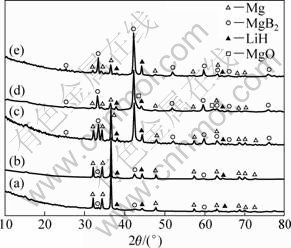
Fig.5 XRD patterns of dehydrogenated 2LiBH4 + MgH2 systems performed under initial dehydrogenation pressures of 1.0×105 Pa (a), 2.0×105 Pa (b), 3.0×105 Pa (c), 4.0×105 Pa (d) and 4.8×105 Pa (e)
3.3 Investigation on influence of dehydrogenation temperature
Figure 6 shows the dehydriding curve of 2LiBH4+ MgH2 system from room temperature to 500 °C under 4.0×105 Pa initial hydrogen pressure. It can be seen that no dehydriding plateau appears after the first desorption step, and hydrogen is rapidly released along with temperature rising. Finally, a dehydriding capacity of 8.49% is obtained within 5 h. The XRD pattern of the dehydrogenated product is shown in Fig.7(a). Unfortunately, a large amount of unexpected Mg metal remained, besides some MgB2 formed. From the relative diffraction intensity of MgB2 to Mg metal in the XRD pattern, we infer that the main proportion of LiBH4 is decomposed independently, and a small amount of LiBH4 retains for reacting with Mg to produce MgB2. It suggests that, although the dehydriding rate is improved, the temperature of 500 °C is too high to suppressing direct decomposition of LiBH4 under 4.0×105 Pa initial hydrogen pressure. The presence of MgH2 in the products probably originated from rehydrogenation of Mg metal during air cooling from 500 °C to room temperature under the hydrogen pressure of 4.0×105 Pa.
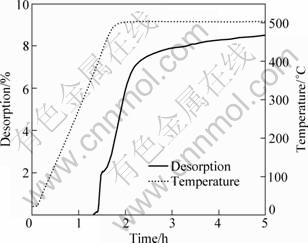
Fig.6 Dehydriding curve of 2LiBH4 + MgH2 system from room temperature to 500 °C under 4.0×105 Pa initial hydrogen pressure and temperature ramping rate of 5 °C/min
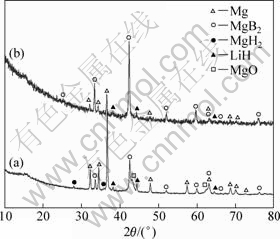
Fig.7 XRD patterns of dehydrogenated 2LiBH4 + MgH2 systems performed under different conditions: (a) RT -500 °C for 5 h; (b) RT -400 °C for 15 h, then increased to 450 °C for 13 h
Figure 8 shows the dehydriding curve of 2LiBH4+ MgH2 system from room temperature to 400 °C then increased to 450 °C under 4.0×105 Pa initial hydrogen pressure. It is found that the dehydriding plateau at 400 °C is quite flat, suggesting that the decomposition of LiBH4 is suppressed significantly. At this time, the amount of hydrogen desorbed almost maintains at 2.8% and no trace of the formation of MgB2 (massive hydrogen is released abruptly) appears until temperature increases to 450 °C, even though dehydriding plateau extends to 15 h at 400 °C. The phase composition after dehydrogenation is given in Fig.7(b). The relative diffraction intensity of MgB2 in the final product, which is similar to that in Fig.2(b), is in agreement with the dehydriding behavior shown in Fig.8. This indicates that the temperature of 400 °C is not high enough to facilitate the formation of MgB2 in 2LiBH4+MgH2 system, though the direct decomposition of LiBH4 is suppressed effectively. As a result, it can be concluded that the temperature of 450 °C is proper for the formation of MgB2 in the 2LiBH4+MgH2 system under 4.0×105 Pa initial dehydrogenation pressure.
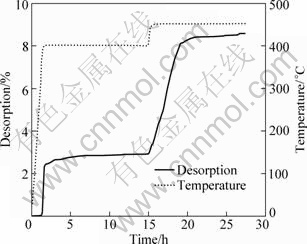
Fig.8 Dehydriding curve of 2LiBH4 + MgH2 system from room temperature to 400 °C and then increased to 450 °C under 4.0×105 Pa initial hydrogen pressure (at 400 °C for 15 h and at 450 °C for 13 h, temperature ramping rate of 5 °C/min)
On the basis of above results, it is found that the dehydrogenation of 2LiBH4+MgH2 system proceeds more completely and more hydrogen desorption capacity can be obtained within a definite time by forming MgB2 than separated decomposition of LiBH4 and MgH2. Actually, the formation process of MgB2 obeys the general features of nucleation, in particular, the effect of supercool or superheat and component concentration on the potency of nucleation: 1) the increase of the supercool degree or superheat degree can enhance the nucleation; 2) the nucleation potency improves with the component concentration of the reactants close to stoichiometric ratio of the product[13-14].
In general, the nucleation rate increases dramatically along with superheat rising, resulting in that the incubation is shortened significantly[15]. According to the above experiment results, it is found that the formation of MgB2 indeed requires an incubation period, exhibiting as a plateau in the dehydriding curve, which is greatly affected by reaction temperature. It shows that elevated temperature promotes the incubation of MgB2, and the ability of incubation is deteriorated at the decreased temperature. Furthermore, it shows that the component concentration of LiBH4 to Mg is maintained more closer to 2:1 during the dehydriding plateau, the incubation process for MgB2 is more favorable, and vice versa. Under the lower hydrogen gas back-pressure, LiBH4 is decomposed quickly, resulting in little LiBH4 remained for the incubation of MgB2. Due to the relatively higher hydrogen gas back-pressure inhibiting the decomposition of LiBH4, there is sufficient LiBH4 for incubation of MgB2. As a result, the component concentration of LiBH4 to Mg is maintained more closer to 2:1 during the dehydriding plateau under relatively higher hydrogen gas back-pressure than the lower hydrogen gas back-pressure. So, it can be seen from Fig.4 that the incubation period is shortened by increasing hydrogen gas back-pressure. Consequently, it is inferred that the incubation period of 1.0×105 Pa or 2.0×105 Pa initial dehydrogenation pressure is much longer than that of 3.0×105 Pa. The reason that the incubation plateau was not observed clearly was that the majority of LiBH4 decomposed independently and only a small amount of LiBH4 reacted with Mg to produce MgB2, which was good consistent with the XRD reflection result. On the basis of above analysis, it is suggested that the plateau of the dehydriding curve relates to the incubation period for nucleation of MgB2, after which it should be the rapid growth of nucleus accompanying with a large amount of H2 released.
At the same time, the results show that none of the involved experiments in this work entirely followed DP(1) to produce only MgB2 rather than Mg. Because MgB2 plays a key role in the reversibility of LiBH4-MgH2 system, the full formation of MgB2 is necessary during the dehydrogenation of 2LiBH4+MgH2 system. In fact, there are two ways to make the dehydrogenation of 2LiBH4+MgH2 system just only follow DP(1): first one is to violently suppress the direct decomposition of LiBH4. Generally, increasing initial dehydrogenation pressure can improve the ability in suppressing the decomposition of LiBH4 at constant temperature. However, excess high pressure will inevitably lead to the decomposition of MgH2 in harsh condition. Thus, the hydrogen pressure applied in the decomposition of LiBH4 should be as low as possible. In this case, it shows that an applied hydrogen pressure of at least 4.0×105 Pa is potentially appropriate to obtain comprehensive ability in yielding MgB2 at 450 °C in 2LiBH4+MgH2 system. Nevertheless, a small sealed reaction volume may be used for dehydrogenation of 2LiBH4+MgH2 system under relative lower initial dehydrogenation pressure or vacuum, through which not only MgH2 decomposes readily but also there is sufficient hydrogen pressure to suppress the direct decomposition of LiBH4 after the decomposition of MgH2. The other way for the full formation of MgB2 is to make the incubation period very short so that the reaction of LiBH4 with Mg occurs quickly before the separated decomposition of LiBH4. Adding nucleating agent or catalysts may be a useful method for that purpose. So, it seems that using a proper small sealed reaction volume with nucleating agent or catalyst is a potentially effective strategy for improving the comprehensive properties of 2LiBH4+MgH2 system, which is being investigated currently.
4 Conclusions
1) The dehydrogenation pathway of 2LiBH4+MgH2 system was investigated carefully. It is found that the dehydrogenation pathway is determined by initial dehydrogenation pressure, which suppresses the direct decomposition of LiBH4 or not.
2) Under relatively higher initial dehydrogenation pressure, LiBH4 reacts with Mg to produce MgB2. However, under relatively lower initial dehydrogenation pressure, LiBH4 is decomposed independently, resulting in no formation of MgB2.
3) The dehydrogenation of 2LiBH4+MgH2 system proceeds more completely and more hydrogen desorption amount can be obtained within a definite time by forming MgB2.
4) The formation process of MgB2 consisting of incubation period and nucleus growth process is proposed. The results show that the formation process of MgB2 is enhanced by increasing initial dehydrogenation pressure at constant temperature. Additionally, elevated temperature could significantly reduce incubation period, while the ability to suppress the decomposition of LiBH4 is deteriorated under a finite hydrogen gas back-pressure.
References
[1] SCHLAPBACH L, Z?TTEL A. Hydrogen-storage materials for mobile applications [J]. Nature, 2001, 414: 353-358.
[2] Z?TTEL A, WENGER P, RENTSCH S, SUNDAN P, MAURON P H, EMMENEGGER C H. LiBH4 a new hydrogen storage material [J]. Journal of Power Sources, 2003, 118(1-2): 1-7.
[3] ORIMO S, NAKAMORI Y, KITAHARA G, MIWA K, OHBA N, TOWATA S, Z?TTEL A. Dehydriding and rehydriding reactions of LiBH4 [J]. Journal of Alloys and Compounds, 2005, 404-406: 427-430.
[4] Z?TTEL A, RENTSCH S, FISCHER P, WENGER P, SUNDAN P, MAURON P H, EMMENEGGER C H. Hydrogen storage properties of LiBH4 [J]. Journal of Alloys and Compounds, 2003, 356-357: 515-520.
[5] VAJO J J, SKEITH S L, MERTENS F. Reversible storage of hydrogen in destabilized LiBH4 [J]. The Journal of Physical Chemistry B, 2005, 109(9): 3719-3722.
[6] VAJO J J, OLSON G. L. Hydrogen storage in destabilized chemical systems [J]. Scripta Materialia, 2007, 56(10): 829-834.
[7] PINKERTON F, MEYER M S, MEISNER G P, BALOGH M P, VAJO J J. Phase boundaries and reversibility of LiBH4/MgH2 hydrogen storage material [J]. The Journal of Physical Chemistry C, 2007, 111(35): 12881-12885.
[8] NAKAGAWA T, ICHIKAWA T, HANADA N, KOJIMA Y, FUJII H. Thermal analysis on the Li-Mg-B-H systems [J]. Journal of Alloys and Compounds, 2007, 446-447: 306-309.
[9] PRICE T E C, GRANT D M, TELEPENI I, YU X B, WALKER G S. The decomposition pathways for LiBD4-MgD2 multicomponent systems investigated by in situ neutron diffraction [J]. Journal of Alloys and Compounds, 2009, 472(1-2): 559-564.
[10] WALKER G S, GRANT D M, PRICE T E C, YU X B, LEGRAND V. High capacity multicomponent hydrogen storage materials: Investigation of the effect of stoichiometry and decomposition conditions on the cycling behaviour of LiBH4-MgH2 [J]. Journal of Power Sources, 2009, 194(2): 1128-1134.
[11] YU X B, GRANT D M, WALKER G S. A new dehydrogenation mechanism for reversible multicomponent borohydride systems—The role of Li-Mg alloys [J]. Chemical Communications, 2006(36): 3906-3908.
[12] B?SENBERG U, DOPPIU S, MOSEGAARD L, BARKHORDARIAN G, EIGEN N, BORGSCHULTE A, JENSEN T R, CERENIUS Y C, GUTEISCH O, KLASSEN T, DORNHEIM M, BORMANN, R. Hydrogen sorption properties of MgH2-LiBH4 composites [J]. Acta Materialia, 2007, 55(11): 3951-3958.
[13] TEN WOLDE P R, FRENKEL D. Enhancement of protein crystal nucleation by critical density fluctuations [J]. Science, 1997, 277(5334): 1975-1978.
[14] VEKILOV P G. Two-step mechanism for the nucleation of crystals from solution [J]. Journal of Crystal Growth, 2005, 275(1-2): 65-76.
[15] THOMAS J J. A new approach to modeling the nucleation and growth kinetics of tricalcium silicate hydration [J]. Journal of the American Ceramic Society, 2007, 90(10): 3282-3288.
寇化秦,肖学章,陈立新,李寿权,王启东
浙江大学 材料科学与工程学系,杭州 310027
摘 要:对2LiBH4+MgH2体系放氢过程中MgB2的形成条件及机理进行研究。结果表明:在较高的4.0×105 Pa初始氢背压下放氢时,会抑制2LiBH4+MgH2体系中LiBH4的自行分解,进而使其与MgH2分解放氢后生成的Mg发生反应生成MgB2,同时在450 °C、9.6 h内释放出9.16%(质量分数)的氢气;而在较低的1.0×102 Pa初始氢背压下放氢时,体系中LiBH4会先行发生自行分解,从而不能与Mg发生反应生成MgB2,在450 °C、5.2 h内只能放出7.91%的氢气。2LiBH4+MgH2体系放氢生成MgB2可以使放氢反应进行得更彻底,并释放出更多的氢气。2LiBH4+MgH2放氢时MgB2的形成过程是一个孕育-长大的过程,随着氢背压的增高,孕育期缩短;而随着反应温度的降低,孕育期延长。
关键词:配位氢化物;LiBH4;MgB2;储氢;形成机理
(Edited by YANG Hua)
Foundation item: Project (2010CB631300) supported by the National Basic Research Program of China; Project (50631020) supported by the National Natural Science Foundation of China; Project (NCET-07-0741) supported by the Program for New Century Excellent Talents in Universities, China; Project (20090101110050) supported by the University Doctoral Foundation of the Ministry of Education, China
Corresponding author: CHEN Li-xin; Tel/Fax: +86-571-87951152; E-mail: lxchen@zju.edu.cn
DOI: 10.1016/S1003-6326(11)60819-4

