芳香族亚砜缓蚀剂对碳钢的腐蚀抑制作用
来源期刊:中南大学学报(自然科学版)2002年第1期
论文作者:钟世安 周春山 蒋新宇
文章页码:32 - 35
关键词:二对胺基苄基亚砜;芳香族亚砜化合物;缓蚀剂; H2SO4
Key words:Bi(p-amido benzyl) sub-sulphone; sulfoxides of aromatic clan compounds; corrosive inhibitor; H2SO4
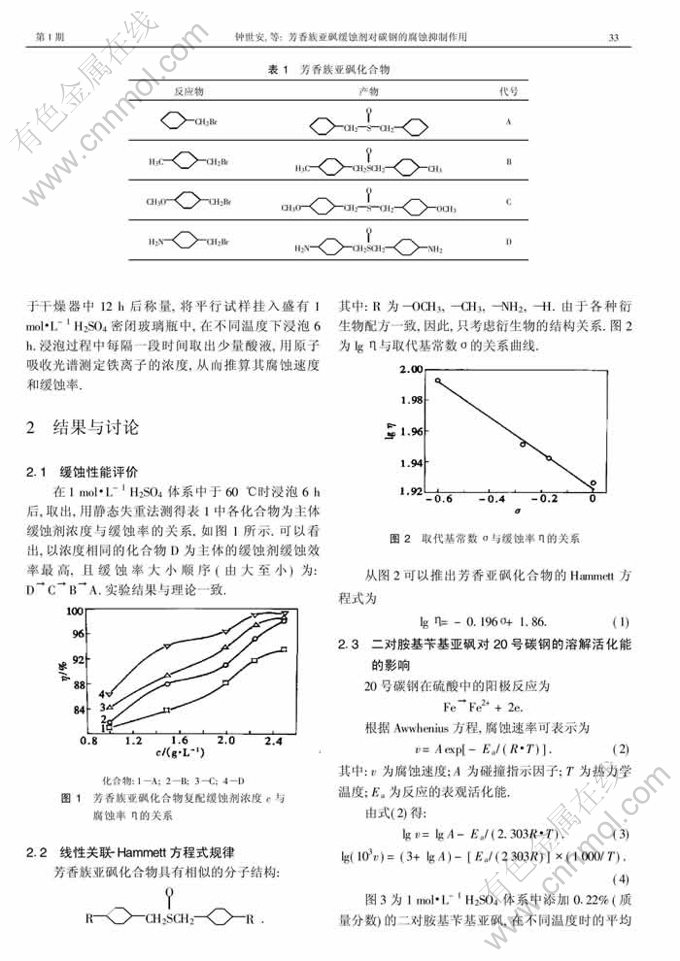
摘 要:按照有机结构-吸附现象-腐蚀性能的线性关系设计合成了几种芳香族亚砜化合物,采用正交设计法添加KI 等配制成相应缓蚀剂,并用失重法评价了它们在H2SO4体系中的缓蚀作用.实验结果表明,芳香亚砜化合物在1 mol ·L-1硫酸介质中对20号碳钢的腐蚀率符合Hammett方程式:lgη=-0.196σ+1.86.在浓度相同的化合物为主体的缓蚀剂中,二对胺基苄基亚砜缓蚀效率最高,且缓蚀率由大到小的顺序为:二对胺基苄基亚砜→二对甲氧基苄基亚砜→二对甲基苄基亚砜→二苄基亚砜;二对胺基苄基亚砜主体缓蚀剂在1 mol·L-1硫酸介质中对20号碳钢的腐蚀率为0.28×10-3kg·m-2·h-1,二对胺基苄基亚砜与I-具有较好的协同效应.
Abstract: According to the direct linear relation principle of organic structure-adsorptive phenomena-corrosive behavior,sulfoxides of aromatic clan compounds are designed and synthesized. Using positive design and appending KI, corresponding corrosive inhibitor was prepared. Their inhibitor effects in H2SO4 system were evaluated with losing weighty method. The result shows that the corrosion inhibitor rate of No.20 charcoal steel in H2SO4containing sulfoxides of aromatic clan is in correspondence with Hammett equation: lgη=-0.196σ+1.86,Bi(p-amido benzyl) sub-sulphone>Bi (p-methyl oxygen benzyl) sub-sulphone>Bi(p-methyl benzyl) sub-sulphone>Bi benzyl sub-sulphone, asm(Bi(p-ami-do benzyl) sub-sulphone)∶m(KI)∶m(superficial)∶m(resolve accelerant)=5∶2∶1∶2. The corrosive rate of Bi(p-amido benzyl) subsulphone inhibitor is 0.28×10-3kg·m-2·h-1 in H2SO4 system for No.20 carbon steel. The experiment result is well-pleasing.