含砷废酸制备亚砷酸铜及其在铜电解液净化中的应用
王 勇,赵攀峰,郑雅杰
(中南大学 冶金科学与工程学院,湖南 长沙,410083)
摘 要:利用含砷废酸制备亚砷酸铜,并将所得亚砷酸铜应用到铜电解液的净化。研究结果表明:使用NaOH溶液调节废酸pH值为6.0时,废酸中Pb,Cu,Fe和Mg杂质的去除率达到90%以上,砷保留率为89.0%;除杂后,加入CuSO4和NaOH溶液,当pH=8,n(Cu)?n(As)=2?1,反应温度为20 ℃,反应时间为1 h时,亚砷酸铜的产率达到98.2%;所得亚砷酸铜为非晶体,其中Cu与As的物质的量比为2.15;当铜电解液中加入20 g/L亚砷酸铜时,铜电解液中Sb和Bi分别从0.65 g/L和0.15 g/L降到0.30 g/L和0.07 g/L,Sb和Bi去除率分别达到53.85%和53.33%。
关键词:废酸;砷;亚砷酸铜;铜电解液;净化
中图分类号:TF803.24; TF803.25 文献标识码:A 文章编号:1672-7207(2007)06-1115-06
Preparation of copper arsenite from waste acid containing arsenic and its application in copper electrolyte purification
WANG Yong, ZHAO Pan-feng, ZHENG Ya-jie
(School of Metallurgical Science and Engineering, Central South University, Changsha 410083, China)
Abstract Copper arsenite prepared from waste acid containing arsenic was applied in copper electrolyte purification. The results show that the removal rates of Pb, Cu, Fe, Mg impurities are up to above 90% and the remaining rate of arsenic in the waste acid is 89.0% when pH value of waste acid is adjusted to 6.0 by NaOH solution. The optimum preparation conditions of copper arsenite are as follows: pH value of solution is 8.0, mole ratio of Cu to As is 2?1, reaction temperature is 20 ℃, and reaction time is 1 h, by adding copper sulphate and NaOH solution after removing impurities. The productivity of copper arsenite with amorphous is 98.2%. Sb concentration decreases from 0.65 g/L to 0.30 g/L, and Bi concentration decreases from 0.15 g/L to 0.07 g/L. The removal rates of Sb and Bi are 53.85% and 53.33%, respectively when copper arsenite concentration in electrolyte is 20 g/L.
Key words: waste acid; arsenic; copper arsenite; copper electrolyte; purification
砷对人体及其他生物有毒并致癌,污水综合排放国家标准GB 8978—1996中规定砷排放质量浓度 ≤0.5 mg/L。铜精矿焙烧时产生的烟气经稀酸洗涤净化后制取硫酸,洗涤后稀酸成为含砷废酸,砷质量浓度一般达到2~10 g/L。
处理含砷废水一般有石灰-铁盐絮凝法、硫化钠沉淀法、萃取和离子交换法。石灰-铁盐法处理含砷废水[1-6]成本低、工艺简单,但产生废渣量大,安全处理费用高,易产生二次污染。萃取[7]、离子交换法[8]工艺复杂、处理成本高。硫化钠沉淀法将砷转化为硫化砷,经氧化、还原、结晶制备三氧化二砷[9-12]。硫化钠沉淀法工艺流程长,处理费用高,但砷回收利用率高,仍被国内外大型冶炼厂广泛采用。
本文作者利用含砷废酸制备得到亚砷酸铜,并将亚砷酸铜成功应用于净化铜电解液,使铜电解液中的Sb和Bi质量浓度分别从0.65 g/L和0.15 g/L降到0.30 g/L和0.07 g/L,Sb和Bi去除率分别达到53.85%和53.33%。
1 实 验
1.1 实验试剂
试剂为:氧化钙(AR),氢氧化钠(工业纯),五水硫酸铜(自制),浓硫酸(AR),含砷废酸,铜电解液。含砷废酸和铜电解液成分分别如表1和表2所示。
表1 含砷废酸的成分
Table 1 Composition of As-containing waste acid ρ/(g?L-1)

表2 铜电解液成分
Table 2 Composition of copper electrolyte ρ/(g?L-1)

1.2 实验步骤
1.2.1 亚砷酸铜的制备
实验取500 mL废酸,使用NaOH溶液调节废酸pH值至2~10,搅拌一定时间后过滤。在滤液中加入适量硫酸铜,充分搅拌使硫酸铜溶解,用NaOH溶液调节溶液pH值为5~10,继续搅拌0.5~4.0 h后过滤,经洗涤、烘干得到绿色亚砷酸铜。其工艺流程如图1所示。

图1 亚砷酸铜制备工艺流程
Fig.1 Flowsheet of copper arsenite prepared from wasted acid
1.2.2 铜电解液的净化
称取烘干后亚砷酸铜加入铜电解液中,在65 ℃反应8 h后过滤,分析滤液中Cu,As,Sb和Bi浓度,计算Sb和Bi杂质脱除率。
1.3 分析与检测
Cu和As采用化学分析法分析,其他元素采用原子吸收光谱法测定。采用X射线衍射仪(D/max-rA,日本理学Rigaku株式会社)分析实验所得样品物相,X射线发射靶为铜靶,管电压为50 kV,管电流为100 mA,2θ为10?~90?。

2 结果及讨论
2.1 pH值对含砷废酸除杂效果的影响
由表1可知,含砷废酸中金属杂质含量较高,实验采用NaOH溶液去除杂质。加入NaOH溶液调节废酸pH值,pH值对废酸中杂质金属离子浓度和去除率的影响分别如表3和表4所示,废酸中砷损失率的影响如图2所示。
表3 pH值对废酸中金属离子浓度的影响
Table 3 Influence of pH value on concentration of metal ions in waste acid
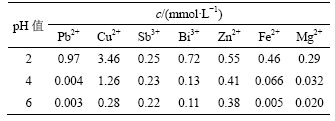
表4 pH值对杂质金属离子去除率的影响
Table 4 Influence of pH value on removal rate of metal impurities
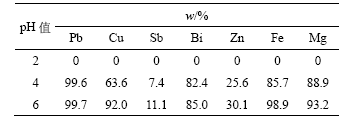
从表3和表4可知,金属离子浓度随pH值增加而降低,金属离子去除率随pH值增加而增加。当pH值为6时,Cu,Pb,Fe和Mg的去除率达到90%以上,Bi的去除率达到85%。当pH值为2时,没有沉淀产生;pH值为4时,产生少量黄色沉淀;pH值为6时,黄色沉淀增多。
废酸中各金属氢氧化物的溶度积(Ksp)[13]如表5 所示。
表5 废酸中金属氢氧化物溶度积Ksp
Table 5 Ksp of metal hydroxide in waste acid

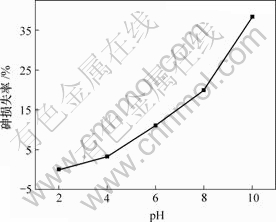
图2 pH值对废酸中砷损失率的影响
Fig.2 Influence of pH value on loss rate of As
根据溶度积计算,pH值为6.0时,废酸中Cu2+和Bi3+产生部分沉淀,其他金属离子在pH≤6.0时均无沉淀产生。溶液中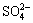 浓度达到0.1 mol/L,硫酸铅的Ksp为1.2×10-8,Pb2+转化为硫酸铅沉淀而被除去。调节pH时,Fe2+被氧化形成溶度积很小的Fe(OH)3沉淀,由于Fe(OH)3吸附与共沉淀作用,使得其他金属离子被除去。
浓度达到0.1 mol/L,硫酸铅的Ksp为1.2×10-8,Pb2+转化为硫酸铅沉淀而被除去。调节pH时,Fe2+被氧化形成溶度积很小的Fe(OH)3沉淀,由于Fe(OH)3吸附与共沉淀作用,使得其他金属离子被除去。
由图2可知,砷损失率随废酸pH值的增加而增加,溶液pH值为2,4,6,8和10时,砷损失率分别为0.0%,3.2%,11.0%,19.9%和38.3%。
综合考虑除杂适宜的pH值为6.0,此时砷保留率为89.0%。
2.2 反应时间对亚砷酸铜产率的影响
将废酸pH值调节为6后加入固体硫酸铜。当硫酸铜与废酸中砷的物质的量比(n(Cu)?n(As))为1?1,反应温度为20 ℃时,反应时间对亚砷酸铜产率的影响如图3所示。
由图3可知,亚砷酸铜产率随反应时间的增加而增大。当反应时间分别为0.5,1.0,2和4 h时,亚砷酸铜产率分别为87.0%,91.1%,91.5%和91.7%。
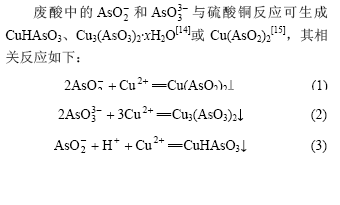
图3 反应时间对亚砷酸铜产率的影响
Fig.3 Influence of reaction time on productivity of copper arsenite
反应中调节溶液pH值为6时,立即产生绿色亚砷酸铜沉淀。随着反应时间的延长,亚砷酸铜产率有所增加。但超过1 h后,亚砷酸铜产率增加不明显,故适宜反应时间为1 h。
2.3 反应溶液pH值对亚砷酸铜产率的影响
上述实验条件不变,反应时间为1 h时,溶液pH值对亚砷酸铜产率的影响如图4所示。
由图4可知,当溶液pH值分别为5,6,8和10时,亚砷酸铜产率分别为39.0%,91.1%,98.2%和98.8%。
溶液中存在以下电离平衡:
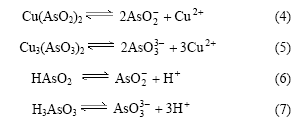
亚砷酸为弱酸,pH值升高,H+浓度降低,电离平衡向右移动。因此,亚砷酸铜产率随pH值的增加而增加。根据亚砷酸铜产率可知适宜pH值为8。
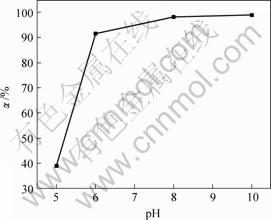
图4 溶液pH值对亚砷酸铜产率的影响
Fig.4 Influence of pH value on productivity of copper arsenite
2.4 铜砷物质的量之比对亚砷酸铜产率的影响
当上述实验条件不变,溶液pH值为8时,n(Cu) ? n(As)对亚砷酸铜产率的影响如图5所示。
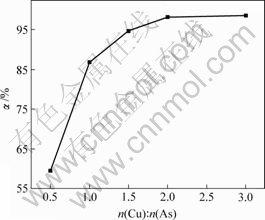
图5 n(Cu)?n(As)对亚砷酸铜产率的影响
Fig.5 Influence of n(Cu):n(As) on productivity of copper arsenite
由图5可知,亚砷酸铜产率随着n(Cu)?n(As)增加而增大,当n(Cu)?n(As)分别为1?2,1?1,3?2,2?1和3?1时,亚砷酸铜产率分别为59.4%,86.8%,94.7%,98.2%和98.6%。
n(Cu)?n(As)越大,溶液中Cu2+浓度越高。因此,废酸中的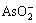 和
和 反应越彻底,亚砷酸铜产率越大。n(Cu)?n(As)=2?1时亚砷酸铜产率已达98.2%,其适宜的n(Cu)?n(As)为2?1。
反应越彻底,亚砷酸铜产率越大。n(Cu)?n(As)=2?1时亚砷酸铜产率已达98.2%,其适宜的n(Cu)?n(As)为2?1。
2.5 反应温度对亚砷酸铜产率的影响
上述实验条件不变,当n(Cu)?n(As)=2?1时反应温度对亚砷酸铜产率的影响如图6所示。
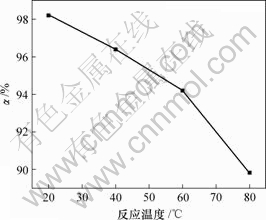
图6 反应温度对亚砷酸铜产率的影响
Fig.6 Influence of reaction temperature on productivity of copper arsenite
由图6可知,亚砷酸铜产率随温度升高而降低。当反应温度分别为20,40,60和80 ℃时,亚砷酸铜的产率分别为98.2%,96.4%,94.2%和89.9%。温度升高,亚砷酸铜溶解度增加,导致了亚砷酸铜产率降低。因此,制备亚砷酸铜的适宜温度为20 ℃。
实验表明,NaOH溶液调节废酸pH值为6除去金属杂质后制备亚砷酸铜适宜的条件是:反应时间为1 h,反应pH为8,n(Cu)?n(As)=2?1,反应温度为20 ℃。在此条件下,2 L含砷废酸制备得到41.7 g亚砷酸铜,产物中Cu和As分别为34.2%和18.7%(质量分数),亚砷酸铜产率为98.2%。
由产物成分可知,产物中Cu与As物质的量比为2.15,因此,反应时以 n(Cu):n(As)=2:1的反应产物主要为Cu3(AsO3)2。对所得产物进行X射线衍射分析,结果如图7所示。可见,所得亚砷酸铜为非晶态。
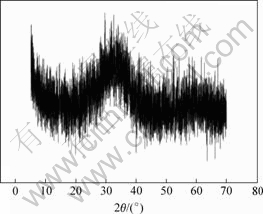
图7 亚砷酸铜XRD谱
Fig.7 XRD pattern of copper arsenite
2.6 亚砷酸铜对铜电解液中Sb和Bi去除率的影响
按照1.2.2步骤进行电解液净化实验,在1 L铜电解液中加入20 g亚砷酸铜,净化后电解液成分如表6所示。
表6 电解液净化结果比较
Table 6 Comparison of electrolyte purification results
ρ/(g?L-1)

由表6可知,采用亚砷酸铜净化铜电解液,铜电解液中的Sb和Bi质量浓度分别从0.65 g/L和0.15 g/L降到0.30 g/L和0.07 g/L,Sb和Bi脱除率分别为53.85%和53.33%。其实验效果与利用三氧化二砷制备亚砷酸铜净化铜电解液结果一致[16]。
铜电解液净化原理复杂,一般在铜电解液中砷、锑、铋作用会发生如下反应[17]:

由于高浓度的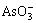 及电解液中活性氧的作用,有利于BiAsO4和SbAsO4沉淀的生成,所以能有效地去除Sb和Bi。
及电解液中活性氧的作用,有利于BiAsO4和SbAsO4沉淀的生成,所以能有效地去除Sb和Bi。
铜电解精炼中,在铜电解液中砷累积可以达到50 g/L。根据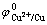 =0.337 V,
=0.337 V,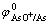 =0.254 V计算判
=0.254 V计算判
断,Cu2+质量浓度为45 g/L时,高砷情况下砷不会析出[18],实践中Cu2+质量浓度≤10 g/L时,砷才会析出。因此,为了降低铜电解液中的Sb和Bi质量浓度,减少漂浮阳极泥的生成,可以通过加入亚砷酸铜提高电解液中As浓度去除Sb和Bi,而阳极溶解的砷进入阳极泥和通过电解净化工段中除去。
采用含砷废酸制备亚砷酸铜[19],不仅使含砷废酸得到处理和资源化,而且可显著地去除电解液中的Sb和Bi杂质,有效地净化铜电解液。
3 结 论
a. 氢氧化钠溶液调节废酸pH值除杂,杂质去除率随pH值升高而增加,砷保留率随pH值升高而降低。pH值为6.0时,废酸中Pb,Cu,Fe和Mg杂质去除率达到90%以上,砷保留率为89.0%。
b. 以含砷废酸为原料制备亚砷酸铜,亚砷酸铜产率随pH值、n(Cu)?n(As)、反应时间的增加而增加,随反应温度升高而降低,制备亚砷酸铜的适宜工艺条件为:pH=8,n(Cu)?n(As)=2?1,反应温度为20 ℃,反应时间为1 h。该条件下亚砷酸铜产率为98.2%,产物中n(Cu)?n(As)为2.15,所得亚砷酸铜为非晶体。
c. 将制得的亚砷酸铜净化铜电解液,当亚砷酸铜加入量为20 g/L时,铜电解液中的Sb和Bi分别从0.65 g/L和0.15 g/L降到0.30 g/L和0.07 g/L,Sb和Bi去除率分别为53.85%和53.33%。
参考文献:
[1] 谢立靖, 邱丽君, 娄 涛, 等. 硫酸生产废水中砷去除方法的研究[J]. 化工环保, 1998, 18(5): 285-291.
XIE Li-jing, QIU Li-jun, LOU Tao, et al. Study on the methods for removal of arsenic in the wastewater from sulphuric acid production[J]. Environmental Protection of Chemical Industry, 1998, 18(5): 285-291.
[2] 何少先, 孙 石, 龚光碧. 净化去除酸性废水中不同价态砷的研究[J]. 环境科学, 1994, 15(4): 44-65.
HE Shao-xian, SUN Shi, GONG Guang-bi. Study on the removal of arsenic in different states of valence from acidic wastewater[J]. Chinese Journal of Environmental Science, 1994, 15(4): 44-65.
[3] Dutre V, Ecasteele C. Solidification/stabilization of hazardous arsenic containing waste from a copper refining process[J]. Journal of Hazardous Materials, 1995, 40: 55-68.
[4] 钟细斌. 硫酸生产中废水治理工艺的改进[J]. 化工环保, 1995, 15(5): 307-312.
ZHONG Xi-bin. Improvement of wastewater treatment process in sulphuric acid production[J]. Environmental Protection of Chemical Industry, 1995, 15(5): 307-312.
[5] 朱义年, 张 华, 梁延鹏, 等. 砷酸钙化合物的溶解度及其稳定性随pH值的变化[J]. 环境科学学报, 2005, 25(12): 1652-1660.
ZHU Yi-nian, ZHANG Hua, LIANG Yan-peng, et al. Dependence of solubility and stability of calcium arsenates on pH value[J]. Acta Scientiae Circumstantiae, 2005, 25(12): 1652-1660.
[6] 张荣良, 丘克强, 谢永金, 等. 铜冶炼闪速炉烟尘氧化浸出与中和脱砷[J]. 中南大学学报: 自然科学版, 2006, 37(1): 73-78.
ZHANG Rong-liang, QIU Ke-qiang, XIE Yong-jin, et al. Treatment process of dust from flash smelting furnace at copper smelter by oxidative leaching and dearsenifying process from leaching solution[J]. Journal of Central South University: Science and Technology, 2006, 37(1): 73-78.
[7] Dapaah A R K, Ayame A. Solvent extraction of arsenic from acid medium using zinc hexamethylenedithiocarbamate as an extractant[J]. Analytica Chimica Acta, 1998, 360(1/3): 43-52.
[8] Jay J A, Blute N K, Hemond H F, et al. Arsenic-sulfides confound anion exchange resin speciation of aqueous arsenic[J]. Water Research, 2004, 38(5): 1155-1158.
[9] 马 伟, 马荣骏, 申殿邦. 硫化法与磁场协同处理含砷废水的研究[J]. 矿冶工程, 1998, 18(3): 44-46.
MA Wei, MA Rong-jun, SHEN Dian-bang. Treatment of as containing wastewater by synergeti use of sulfidation and magnetic field effect[J]. Mining and Metallurgical Engineering, 1998, 18(3): 44-46.
[10] 刘昌勇. 贵溪冶炼厂亚砷酸生产工艺[J]. 有色冶炼, 1998, 27(2): 8-10.
LIU Chang-yong. Arsenious acid production in Guixi Smelter[J]. Non-Ferrous Smelting, 1998, 27(2): 8-10.
[11] 陈维平, 李仲英, 边可君, 等. 湿式提砷法在处理工业废水及废渣中的应用[J]. 中国环境科学, 1999, 19(4): 310-312.
CHEN Wei-ping, LI Zhong-ying, BIAN Ke-jun, et al. Application of wet-method for extracting arsenic in treating industrial wastewater and residues[J]. China Environmental Science, 1999, 19(4): 310-312.
[12] 田文增, 陈白珍, 仇勇海. 有色冶金工业含砷物料的处理及利用现状[J]. 湖南有色金属, 2004, 20(6): 11-15.
TIAN Wen-zeng, CHEN Bai-zhen, QIU Yong-hai. Review of as-containing materials treatment and utilization in nonferrous metallurgy industry[J]. Hunan Nonferrous Metals, 2004, 20(6): 11-15.
[13] 迪安 J A. 兰氏化学手册[M]. 北京: 科学出版社, 1991.
Dean J A. Lange’s handbook of chemistry[M]. Beijing: Science Press, 1991.
[14] 陈寿椿. 重要无机化学反应[M]. 3版. 上海: 上海科学技术出版社, 1994: 1379.
CHEN Shou-chun. Important inorganic chemical reactions[M]. 3rd ed. Shanghai: Shanghai Science and Technology Press, 1994: 1379.
[15] Nishimura T, Itoh C T, Tozawa K. Stabilities and solubility’s of metal arsenates and arsenates in water and effect of sulfate ions on their solubility’s[C]//Reddy R G, Hendrix J L, Queneau P B. Arsenic Metallurgy Fundamentals and Applications. Reno: University of Nevada-Reno, 1988: 77-98.
[16] 郑雅杰, 肖发新, 王 勇. 亚砷酸铜的制备及应用: 中国, 200610031980.7[P]. 2006-07-19.
ZHENG Ya-jie, XIAO Fa-xin, WANG Yong. The preparation and application of arsenic copper: China, 200610031980.7[P]. 2006-07-19.
[17] 文 燕, 张 源, 张胜树. 铜电解过程中杂质分配的控制[C]//全国铜镍钴生产技术、装备、材料及市场研讨会. 北京: 中国有色金属学会, 2003: 93-97.
WEN Yan, ZHANG Yuan, ZHANG Sheng-shu. Control of impurities in copper electrolysis[C]//Symposium of production technology, equipment, material and market of copper, nickel and cobalt in China. Beijing: Chinese Nonferrous Metal Society, 2003: 93-97.
[18] 朱祖泽, 贺家齐. 现代铜冶金学[M]. 北京: 科学出版社, 2003.
ZHU Zu-ze, HE Jia-qi. Modern metallurgy of copper[M]. Beijing: Science Press, 2003.
[19] 郑雅杰, 王 勇, 赵攀峰.一种利用含砷废水制备亚砷酸铜和砷酸铜的方法: 中国, 200610032456.1[P]. 2006-10-25.
ZHENG Ya-jie, WANG Yong, ZHAO Pan-feng. A method of producing arsenite copper and arsenate copper from waste acid contained As: China, 200610031980.7[P]. 2006-10-25.
收稿日期:2007-03-05;修回日期:2007-04-25
基金项目:广东省产学研重点项目(2007A090302068);湖北大冶有色金属有限公司项目(2006KJ11)
作者简介:王 勇(1961-),男,江苏睢宁人,博士研究生,从事湿法冶金、矿物加工研究
通信作者:郑雅杰,男,教授,博士;电话:0731-8836285;E-mail:zzyyjj01@yahoo.com.cn