锰方解石的盐酸浸出及四氧化三锰的制备
杨志超1,冯雅丽1,李浩然2,刘欣伟1
(1. 北京科技大学 土木与环境工程学院,北京,100083;
2. 中国科学院过程工程研究所 生化工程国家重点实验室,北京,100190)
摘要:对锰方解石的盐酸浸出行为进行研究并采用石灰乳法制备四氧化三锰;采用X线衍射对产品Mn3O4进行表征,考察液固比、催化剂用量、反应温度、浸出时间和搅拌速度等因素对锰浸出率的影响,并从动力学角度分析浸出行为。为了提高产品四氧化三锰的纯度,采用正交实验研究石灰乳浓度为1 mol/L 时,Mn2+浓度、反应温度、石灰乳加料速度以及反应陈化时间对氢氧化锰纯度的影响。研究结果表明:锰浸出率随温度和搅拌速度的增大而增大;催化剂可在一定程度上缩短反应时间;锰方解石矿的浸出过程为扩散-化学反应混合控制过程;当氯化锰浓度为0.5 mol/L,反应温度为80 ℃,陈化时间为4 h,加料速度为6 mL/min时,氢氧化锰中锰质量分数可达60%以上,最终产品四氧化三锰中锰质量分数为70.42%。
关键词:锰方解石;石灰乳;氢氧化锰;四氧化三锰;浸出;动力学
中图分类号:TF803.24 文献标志码:A 文章编号:1672-7207(2013)01-0025-07
Hydrochloric acid leaching of manganocalcite and preparation of manganese tetraoxide
YANG Zhichao1, FENG Yali1, LI Haoran2, LIU Xinwei1
(1. School of Civil and Environment Engineering, University of Science and Technology Beijing, Beijing 100083, China;
2. State Key Laboratory of Biochemical Engineering, Institute of Process Engineering,
Chinese Academy of Sciences, Beijing 100190, China)
Abstract: The processes of preparing manganese from manganocalcite by hydrochloric acid-leaching and technological conditions of the preparation of manganese tetraoxide by lime-brine method were studied, and the crystal configuration of manganese tetraoxide was characterized by X-ray diffraction. The effects of liquid-solid ration, content of catalysts, reaction temperature, reaction time and stir speed on leaching rate were investigated, and the leaching process was analyzed by kinetics. In order to improve the purity of manganese hydroxide product, manganese ion concentration, reaction temperature, feeding speed and aging time were investigated through orthogonal experiments when the concentration of lime milk was 1 mol/L. The results show that with the increase of the reaction temperature and stir speed, the manganese leaching rate increases. Catalysts can shorten the reaction time to some extent, and the reaction is controlled by diffusion-chemical reaction hybrid controlled process. Above 60% of manganese in manganese hydroxide is achieved when feeding speed is 6 mL/min with manganese ion concentration of 0.5 mol/L at 80 ℃ for 4 h. The mass fraction of manganese in the product is 70.42%.
Key words: manganocalcite; lime milk; manganese hydroxide; manganese tetraoxide; leaching; kinetics
我国锰矿类型以碳酸锰为主,碳酸锰质量约占锰矿总储量的73%,其品位低、杂质含量高、粒度小、选别难度大,部分富锰矿石在利用时仍需要工业加工[1-2]。国内外许多学者对碳酸锰矿石的浸取进行了大量的研究工作,如:李赋屏等[3-5]利用铵盐在一定温度下焙烧碳酸锰,以热水浸取焙烧料,锰浸出率可达90%以上;刘云等[6]认为软锰矿与菱锰矿混合矿浆烟气脱硫不但能有效脱除烟气中的二氧化硫,而且可以得到较高的锰浸出率;孟运生等[7]研究了以菌生黄铁矿浸矿剂对锰矿石的浸出试验,锰浸取率可达60%以上;Elsherief[8]采用循环伏安法,在硫酸介质中于反应槽通过电流作用浸取锰矿;Momade等[9]研究了氧化锰矿在甲醇-硫酸水溶液中的还原浸出,在160 ℃时,氧化锰矿石在体积分数为40%甲醇与0.3 mol/L硫酸溶液中浸出2 h,锰浸出率可达98%。上述方法存在能耗较大、成本较高、污染环境、生产效率低、浸出条件苛刻等不足。到目前为止,四氧化三锰的制备常采用氨水介质中用空气氧化MnSO4中Mn2+制得,并通过控制溶液pH,降低产品中硫、钙、镁的含量[10],采用石灰乳法制备氢氧化锰的相关报道较少。本文作者针对锰方解石含钙高的特点,采用传统湿法冶锰技术即直接酸浸法,利用盐酸浸取锰和钙,石灰乳法生产四氧化三锰和氯化钙,以期找到一种新的工艺简单、生产成本低、无环境污染、产品纯度高的碳酸锰矿利用方法,为同类锰矿石利用的生产实践起参考作用。
1 材料与方法
1.1 试验原料
试验用矿样取自贵州省六盘水市某锰矿区,其化学多元素分析和X线衍射分析结果见表1和图1。分析表明:该锰方解石主要矿物组分为CaMn(CO3)2,CaCO3和Mn3O4,主要脉石矿物为SiO2,并含有少量的MgO和Al2O3等。
表1 矿样多元素化学分析结果(质量分数)
Table 1 Chemical compositions of samples %
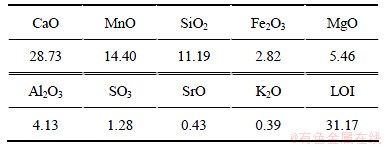

图1 矿样的X线衍射图
Fig.1 X-ray diffraction pattern of sample
1.2 试验方法
(1) 将矿石破碎、研磨至粉状,过筛孔径为0.150 mm的筛备用。称取30 g矿粉于250 mL烧杯中,加入体积分数为30%的盐酸和催化剂H,将烧杯置于设定温度的恒温水浴锅中,搅拌反应一定时间后,将矿浆快速过滤、洗涤,取滤液测定锰含量,计算锰浸出率。试验所用试剂均为化学纯,催化剂H为强氧化性试剂。
(2) 将一定量的生石灰加入到温度为80~90 ℃的热水中消化2 h,并陈放1~2 h, 过75 μm筛,加水稀释成1 mol/L的Ca(OH)2乳液。浸出液净化后,按MnCl2 浓度调配至一定浓度,在设定的温度、加料速度和搅拌条件下加入适量Ca(OH)2乳液,得到Mn(OH)2沉淀;陈化一定时间后过滤,且用去离子水充分洗涤沉淀至洗水中无氯离子检出。将上述滤饼转移至反应器中,按液固比4:1加水搅拌,于70 ℃水浴中鼓空气,控制pH<7.3;反应结束后,过滤,洗涤,烘干,得产品四氧化三锰。
2 结果与讨论
2.1 浸出条件试验
试验主要采用单因素试验的研究方法,考察液固比、催化剂用量、反应温度、浸出时间、搅拌速度等因素对锰浸出率的影响。
2.1.1 液固比与催化剂用量对锰浸出率的影响
液固比与催化剂用量对锰浸出率的影响如图2所示。浸出条件如下:温度为25 ℃,搅拌速度为400 r/min,反应时间为2 h。从图2可以看出:液固比对锰浸出率的影响与催化剂的影响相比较明显;当催化剂用量为0.67 mL/g,液固比为0.75,1.46和2.00 mL/g时,锰浸出率分别为95.03%,97.20%和99.80%,为便于浸出液后续处理,选择液固比为1.46 mL/g;当液固比为1.46,催化剂用量从0~0.67 mL/g变化时,浸出率只提高了2.20%。因此,为了缩短反应时间和净化浸出液,催化剂用量为0.27 mL/g。
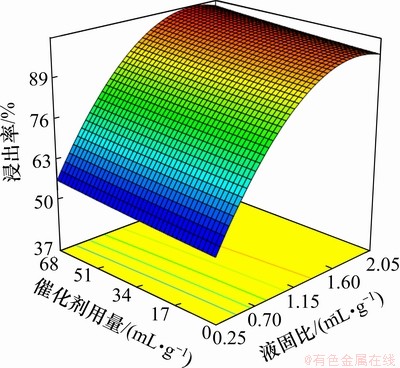
图2 液固比与催化剂用量对锰浸出率的影响
Fig.2 Effect of leaching liquid to solid ration and quantity of catalyst on leaching rate of manganese
2.1.2 反应温度对锰浸出率的影响
在液固比为1.46 mL/g,催化剂用量为0.27 mL/g,搅拌速度为400 r/min时,于不同温度下进行浸出反应,结果如图3所示。从图3可见:随着反应温度的升高,锰的浸出率不断增加,在最初的1 h内,锰浸出率极大,达到95%以上。
2.1.3 搅拌速度与反应时间对锰浸出率的影响
搅拌速度与反应时间对锰浸出率的影响如图4所示。反应条件如下:液固比为1.46 mL/g,催化剂用量为0.27 mL/g,温度为25 ℃。从图4可知:浸出时间对浸出率有较大影响,搅拌速度与锰浸出率关系不大。这表明反应产物MnCl2在溶液和锰方解石颗粒表面的扩散速度较快,反应过程基本消除外扩散阻力的影响,为保证搅拌速度的一致性,选用搅拌速度为400 r/min。
2.2 浸出动力学分析
锰方解石盐酸浸出属于液-固相反应,采用激光粒度分析仪测量浸出渣粒度与反应原料粒度,发现渣颗粒度有所降低,但未产生泥化现象。在浸出过程中,尚未发生反应的固体反应物被酸不溶物包裹,形成了多孔的残留物层,浸出剂必须通过残留层扩散才能与未反应的固相进一步反应,故浸出过程可用收缩未反应核模型描述[11-12]。从图4可见:当搅拌速度达到400 r/min时,提高转速对浸出率影响不大,这种强制性扩散消除了外扩散对浸出过程的限制作用。据研究,碳酸锰矿石浸出反应受化学反应和内扩散混合控制,因此,用方程
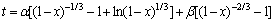
描述锰方解石动力学模型[13]。其中: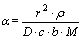 ;
;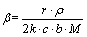 ;r为颗粒初始半径;
;r为颗粒初始半径; 为锰方解石密度;D为有效扩散系数;c为盐酸初始浓度;b为锰方解石反应系数;M为锰方解石相对分子质量;k为反应速率常数;x为锰浸出率。令
为锰方解石密度;D为有效扩散系数;c为盐酸初始浓度;b为锰方解石反应系数;M为锰方解石相对分子质量;k为反应速率常数;x为锰浸出率。令
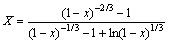

将图3(b)中数据按 进行回归,得到不同温度下的
进行回归,得到不同温度下的 和
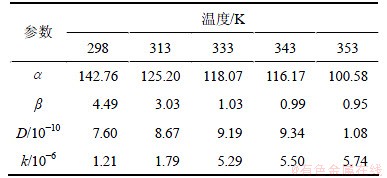
和 ,并计算出D和k,如表2所示。其中:r=0.01 cm。
,并计算出D和k,如表2所示。其中:r=0.01 cm。

图3 温度对锰浸出率的影响
Fig.3 Effect of temperature on leaching rate of manganese

图4 浸出时间与搅拌速度对锰浸出率的影响
Fig.4 Effect of reaction time and stirring speed onleaching rate of manganese
表2 模型回归值
Table 2 Regressed value of kinetic model
根据Arrhenius公式[14],D和k可用通式表示为: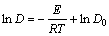 和
和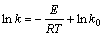 。将表2中D和k以ln A-1/T作图(见图5),从而可求得:D0=4.201×10-9;ED=4.167 kJ/mol;k0=0.083 96;Ek=27.60 kJ/mol。
。将表2中D和k以ln A-1/T作图(见图5),从而可求得:D0=4.201×10-9;ED=4.167 kJ/mol;k0=0.083 96;Ek=27.60 kJ/mol。
由表观反应活化能数值判断,该反应由化学反应和内扩散混合控制[15-16]。根据以上数据可以导出t的具体表达式为:
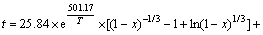

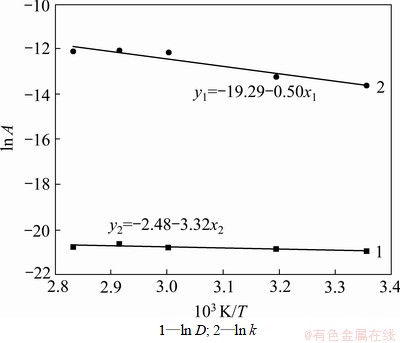
图5 ln A与1/T的关系
Fig.5 Relationship between ln A and 1/T measured
为了验证动力学模型的准确度,图6给出了35 ℃和50 ℃时浸出率与浸出时间的关系。从图6可以看出实验结果与模型计算结果较吻合,表明以上动力学方程能较好地描述锰方解石盐酸浸出行为,进一步证实了反应由化学反应与扩散混合控制。
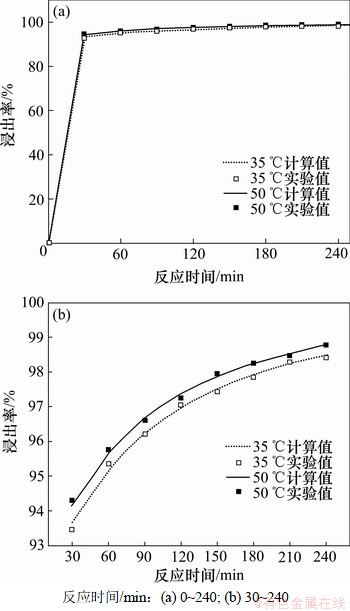
图6 浸出率与浸出时间的关系
Fig.6 Relationship between leaching efficiency and time
2.3 四氧化三锰的制备
2.3.1 浸出液的净化
锰方解石经盐酸浸取锰时,钙、铁、硅、铝、镁等杂质也不同程度地进入溶液,它们会对锰氧化物产品的纯度产生影响,通常用共沉淀法除去。由于本实验采用石灰乳法沉淀Mn2+,在沉淀过程中钙镁会形成副产品,因此,在浸出液净化时可不必除钙镁。当浸出液pH 约为3.5时,加入软锰矿或双氧水使Fe2+氧化为Fe3+,再用石灰乳将溶液的pH 调至6.5,生成Fe(OH)3和Al(OH)3沉淀,二氧化硅以硅酸形式析出,煮沸过滤即可除去铁、铝、硅离子。对于重金属离子,采用加硫化钠的方法,使重金属离子与S2-反应生成金属硫化物沉淀。最后,仍需向净化浸出液中加入少量石灰乳液,生成少量Mn(OH)2胶体沉淀。该沉淀具有较大的比表面积,能吸附包裹Fe(OH)3和Al(OH)3等微细杂质,同时,它又可能被Fe2+等离子同晶置换形成固溶体,进一步净化浸出液。
2.3.2 影响四氧化三锰纯度的因素正交实验
在石灰乳与MnCl2反应生成氢氧化锰时,Mn2+浓度、反应温度、石灰乳加料速度以及反应后陈化时间都会影响氢氧化锰沉淀的纯度,进而影响到四氧化三锰的纯度。以浓度为1 mol/L的石灰乳进行实验,设计正交实验如表3所示,正交实验结果如表4所示。
从表4可见:4个因素中,对产品锰含量影响由大至小的因素依次为氯化锰浓度、温度、陈化时间和石灰乳浓度。最佳反应条件如下:氯化锰浓度为0.5 mol/L,反应温度为80 ℃,陈化时间为4 h,加料速度为6 mL/min。
采用SPSS软件对正交结果进行方差分析,结果如表5所示。
方差F检验结果表明:正交实验因子显著性从大到小依次为浓度、温度、陈化时间和加料时间,与极差分析结果一致。重复正交实验A1B3D3 C1 3次,锰质量分数分别为60.23%,60.19%和59.44%,结果较理想。
表3 正交实验表
Table 3 Orthogonal experimental design
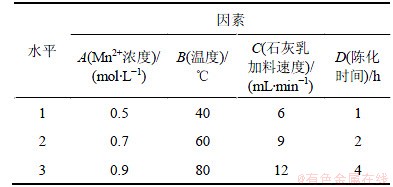
表4 正交实验结果
Table 4 Orthogonal experimental results

表5 方差分析表
Table 5 Analysis of variance table

3 产品分析
在温度为80 ℃、浓度为0.5 mol/L的氯化锰溶液中以 6 mL/min 的速度加入1 mol/L 的新鲜石灰乳, 搅拌陈化反应4 h,终点pH=10.8,沉淀物洗涤至滤液中无Cl-为止。将上述滤饼转移至反应器中,按液固比 4:1加水搅拌,于70 ℃水浴,鼓空气,控制pH<7.3。反应结束后,过滤、洗涤、烘干,得产品四氧化三锰。取样品进行X线衍射与多元素化学分析,结果见图7与表6。
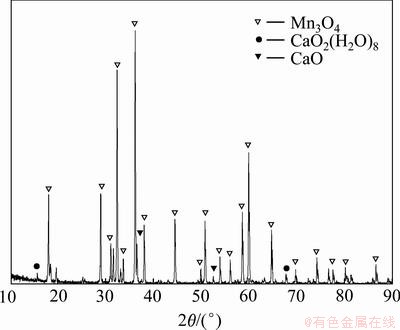
图7 四氯化三锰产品的X线衍射图
Fig.7 X-ray diffraction patterns of manganese tetraoxide
表6 四氧化三锰多元素化学分析结果(质量分数)
Table 6 Chemical compositions of manganese tetraoxide %

从图7可看出:产品Mn3O4特征峰尖锐且比较窄,说明产品结晶性良好,粒径比较大;与Mn3O4标准 XRD 图谱进行比较,结果一致性较高,仅有少量杂峰,可见产品纯度较高。化学多元素分析也表明产品锰质量分数可达 70.42%,但产品中含Cl和Ca质量分数较高,可能是反应过程中有未反应的石灰乳被包裹和不易被氧化的碱式氯化锰生成所致。
4 结论
(1) 在液固比为1.46 mL/g、催化剂用量为0.27 mL/g、温度为25 ℃、搅拌速度为400 r/min、浸出反应为4 h时,锰方解石锰浸出率可达98%以上;在反应最初的1 h内,锰浸出率极大,达到95%以上,说明盐酸溶液可以很好地将锰方解石中的锰浸出。
(2) 对浸出过程进行的动力学研究,证实了浸出过程由内扩散与化学反应混合控制,浸出动力学方程为:
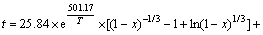
 ;
;
其表观活化能ED=4.167 kJ/mol,Ek=27.60 kJ/mol。
(3) 在石灰乳与 MnCl2 反应生成氢氧化锰时,Mn2+浓度、反应温度、石灰乳加料速度以及反应后陈化时间都会影响到氢氧化锰沉淀的纯度,进而影响到四氧化三锰的纯度。生成氢氧化锰的最佳反应条件为:氯化锰浓度 0.5 mol/L,反应温度80 ℃,陈化时间4 h,加料速度6 mL/min。
(4) 产品Mn3O4 XRD图中特征峰窄而尖,说明产品结晶良好,粒径较大。产品中Cl和CaO质量分数较高,是制约石灰乳法制备高纯度四氧化三锰的因素。
参考文献:
[1] 张泾生, 周光华. 我国锰矿资源及选矿进展评述[J]. 中国锰业, 2006, 24(1): 1-5.
ZHANG Jingsheng, ZHOU Guanghua. A review of manganese ore resources in China and its processing technology progress[J]. China’s Manganese Industry, 2006, 24(1): 1-5.
[2] 周柳霞. 我国锰矿山的开采现状及问题与建议[J]. 中国锰业, 2000, 18(1): 6-9.
ZHOU Liuxia. Mining conditions and problems about China’s Mn mines and some proposals[J]. China’s Manganese Industry, 2000, 18(1): 6-9.
[3] 李赋屏, 朱国才, 田君. 从低品位碳酸锰矿石中富集回收锰的绿色化学工艺研究[J]. 矿产与地质, 2005, 19(1): 93-96.
LI Fuping, ZHU Guocai, TIAN Jun. A green chemical process of enriching and recovering Mn from low grade ore of manganese carbonate[J]. Mineral Resources and Geology, 2005, 19(1): 93-96.
[4] 朱国才, 李赋屏, 肖明贵. 采用硫酸铵焙烧方法从低品位碳酸锰矿中富集回收锰[J]. 桂林工学院学报, 2005, 25(4): 534-537.
ZHU Guocai, LI Fuping, XIAO Minggui. Process of enriching and recovering Mn by roasting the low-grade manganese carbonate ore with ammonium sulfate[J]. Journal of Guilin Institute of Technology, 2005, 25(4): 534-537.
[5] 靳晓珠, 杨仲平, 陈祝炳, 等. 低品位碳酸锰矿铵盐焙烧富锰工艺研究[J]. 中国锰业, 2006, 24(1): 28-29.
JIN Xiaozhu, YANG Zhongping, CHEN Zhubing, et al. A study on enriching manganese by roasting low-grade manganese carbonate ore with ammonium salt[J]. China’s Manganese Industry, 2006, 24(1): 28-29.
[6] 刘云, 孙峻, 胡响响, 等. 菱锰矿与软锰矿混合矿浆烟气脱硫研究[J]. 中国锰业, 2008, 26(4): 19-23.
LIU Yun, SUN Jun, HU Xiangxiang, et al. Study on flue gas desulfurization with rhodochrosite and pyrolusite pulp[J]. China’s Manganese Industry, 2008, 26(4): 19-23.
[7] 孟运生, 徐晓军, 王吉坤. 贫锰矿细菌浸出试验研究[J]. 湿法冶金, 2002, 21(4): 184-187.
MENG Yunsheng, XU Xiaojun, WANG Jikun. Research on bacteria leaching of low-grade manganese ore[J]. Hydrometallurgy of China, 2002, 21(4): 184-187.
[8] Elsherief A E. A study of the electroleaching of manganese ore[J]. Hydrometallurgy, 2000, 55(3): 311-326.
[9] Momade F WY, Momade Z G. Reductive leaching of manganese oxide ore in aqueous methanol-sulphuric acid medium[J]. Hydrometallurgy, 1999, 51(1): 103-113.
[10] 邹兴, 孙宁磊, 王国承. 用空气直接氧化游离二价锰离子制备高纯四氧化三锰[J]. 北京科技大学学报, 2007, 29(12): 1250-1253.
ZOU Xin, SUN Ninglei, WANG Guocheng. Preparation of trimanganese tetroxide by air oxidizing free divalent manganese ions[J]. Journal of University of Science and Technology Beijing, 2007, 29(12): 1250-1253.
[11] 朱炳辰. 化学反应工程[M]. 北京: 化学工业出版社, 1993: 173-188.
ZHU Bingchen. Chemical reaction engineering[M]. Beijing: Chemical Industry Press, 1993: 173-188.
[12] 冯其明, 邵延海, 欧乐明, 等. 废催化剂焙烧水浸渣中硫酸浸取钴的动力学研究[J]. 中南大学学报: 自然科学版, 2010, 41(1): 21-26.
FENG Qiming, SHAO Yanhai, OU Leming, et al. Leaching kinetics of cobalt from roasting-dissolving residue of spent catalyst with sulfuric acid[J]. Journal of Central South University: Science and Technology, 2010, 41(1): 21-26.
[13] LI Minting, WEI Chang, QIU Shuang. Kinetics of vanadium dissolution from black shale in pressure acid leaching[J]. Hydrometallurgy, 2010, 104(2): 193-200.
[14] 莫鼎成. 冶金动力学[M]. 长沙: 中南工业大学出版社, 1987: 304-306.
MO Dingcheng. Metallurgy kinetics[M]. Changsha: Central South University of Technology Press, 1987: 304-306.
[15] Souza A D, Pina P S, Lima E V O, et al. Kinetics of sulphuric acid leaching of a zinc silicate calcine[J]. Hydrometallurgy, 2007, 89(3/4): 337-345.
[16] Lozano L J, Juan D. Leaching of vanadium from spent sulphuric acid catalysts[J]. Minerals Engineering, 2001, 14(5): 543-546.
(编辑 陈灿华)
收稿日期:2011-12-11;修回日期:2012-02-27
基金项目:国家自然科学基金资助项目(21176026,21176242);国家高技术研究发展计划(“863”计划)项目(2012AA062401)
通信作者:冯雅丽(1967-),女,北京人,博士,教授,从事矿物加工研究;电话:010-62311181;E-mail: ylfeng126@126.com