Microwave hydrothermal synthesis, characterization and excellent uranium adsorption properties of CoFe2O4@rGO nanocomposite
来源期刊:中南大学学报(英文版)2021年第7期
论文作者:易兵 伍水生 兰东辉 张晓文 黄毅 邓兴红 区泽棠
文章页码:1955 - 1965
Key words:CoFe2O4; graphene; uranium; adsorption properties
Abstract: To improve the adsorption performance and simplify uranium separation from aqueous media in post-treatment processes, a magnetic CoFe2O4@rGO composite was synthesized by microwave-hydrothermal method. The results of XRD, Raman, TEM/HRTEM, FTIR, BET and VSM characterization show that spinel-type cobalt ferrite CoFe2O4 nanoparticles ca. 13.4 nm in size are dispersedly anchored on the graphene sheet, and the saturation magnetization of the nanocomposite is 46.7 mA/(m2·g). The effects of different pH, initial concentration and other conditions on uranium adsorption capacity were investigated, and adsorption kinetics equations were fitted to determine the adsorption behaviour of uranium on CoFe2O4@rGO in simulated uranium-containing seawater. It was observed that the uranium adsorption capacity of CoFe2O4@rGO composite at pH=5 is 127.6 mg/g, which is 1.31 and 2.43 times that of rGO and pure CoFe2O4. The adsorption process conforms to Langmuir and quasi-second-order kinetic model. The excellent adsorption performance of CoFe2O4@rGO makes it potentially useful in the treatment of uranium-polluted water.
Cite this article as: WU Shui-sheng, LAN Dong-hui, ZHANG Xiao-wen, HUANG Yi, DENG Xing-hong, AU Chak-tong, YI Bing. Microwave hydrothermal synthesis, characterization and excellent uranium adsorption properties of CoFe2O4@rGO nanocomposite [J]. Journal of Central South University, 2021, 28(7): 1955-1965. DOI: https://doi.org/ 10.1007/s11771-021-4744-4.

J. Cent. South Univ. (2021) 28: 1955-1965
DOI: https://doi.org/10.1007/s11771-021-4744-4
WU Shui-sheng(伍水生)1, LAN Dong-hui(兰东辉)1, ZHANG Xiao-wen(张晓文)2,HUANG Yi(黄毅)3, DENG Xing-hong(邓兴红)1, AU Chak-tong(区泽棠)1, YI Bing(易兵)1
1. Hunan Provincial Key Laboratory of Environmental Catalysis & Waste Recycling, School of Materials and Chemical Engineering, Hunan Institute of Engineering, Xiangtan 411104, China;
2. School of Resource & Environmental and Safety Engineering, University of South China,Hengyang 421001, China;
3. School of Management, Hunan Institute of Engineering, Xiangtan 411104, China
 Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2021
Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2021
Abstract: To improve the adsorption performance and simplify uranium separation from aqueous media in post-treatment processes, a magnetic CoFe2O4@rGO composite was synthesized by microwave-hydrothermal method. The results of XRD, Raman, TEM/HRTEM, FTIR, BET and VSM characterization show that spinel-type cobalt ferrite CoFe2O4 nanoparticles ca. 13.4 nm in size are dispersedly anchored on the graphene sheet, and the saturation magnetization of the nanocomposite is 46.7 mA/(m2·g). The effects of different pH, initial concentration and other conditions on uranium adsorption capacity were investigated, and adsorption kinetics equations were fitted to determine the adsorption behaviour of uranium on CoFe2O4@rGO in simulated uranium-containing seawater. It was observed that the uranium adsorption capacity of CoFe2O4@rGO composite at pH=5 is 127.6 mg/g, which is 1.31 and 2.43 times that of rGO and pure CoFe2O4. The adsorption process conforms to Langmuir and quasi-second-order kinetic model. The excellent adsorption performance of CoFe2O4@rGO makes it potentially useful in the treatment of uranium-polluted water.
Key words: CoFe2O4; graphene; uranium; adsorption properties
Cite this article as: WU Shui-sheng, LAN Dong-hui, ZHANG Xiao-wen, HUANG Yi, DENG Xing-hong, AU Chak-tong, YI Bing. Microwave hydrothermal synthesis, characterization and excellent uranium adsorption properties of CoFe2O4@rGO nanocomposite [J]. Journal of Central South University, 2021, 28(7): 1955-1965. DOI: https://doi.org/ 10.1007/s11771-021-4744-4.
1 Introduction
As an important strategic nuclide, uranium plays a pivotal role in nuclear applications. With the development of nuclear-related technologies, there is a large amount of radioactive wastewater due to uranium contamination. It is hence of great significance to develop environment-benign methods that are low cost and highly efficient for the adsorption and separation of uranium. The current approaches for treating uranium-containing wastewater include flocculation [1], biological reduction and precipitation [2], reverse osmosis [3], ion exchange [4] and adsorption method [5, 6]. Among the above methods, adsorption has the advantages of low cost, easy operation, no need for large equipment, high uranium removal rate, and no secondary pollution. It is known that the type and morphology of adsorbents have a significant effect on adsorption efficiency. Adsorbents such as clay minerals [7], hydrotalcite [8, 9], metal oxides [10], resin [11] and MOF [6] have long been used for the treatment of uranium in water media worldwide. Nonetheless, shortcomings such as high preparation cost and/or low adsorption efficiency limit the use of these adsorbents, and there is still an urgent need for the development of practical materials for uranium adsorption.
With ultra-high surface area, rich functional groups, and good chemical stability, graphene has high potential in wastewater treatment [9]. However, due to strong van der Waals interaction, there is severe agglomeration of graphene in water, resulting in the loss of effective surface area, and hence reduction of adsorption capacity [10]. Furthermore, because it is not easy to separate graphene from the water phase, there could be secondary pollution. In recent years, magnetic ferrite spinel-graphene composites were used as effective adsorbents that can be rapidly separated by an external magnetic field [11, 12]. In addition, the magnetic nanoparticles can help prevent the aggregation of graphene layers, leading to improved adsorption performance. For example, WU et al [13] prepared magnetic polysaccharide/graphene oxide@Fe3O4 gel beads for efficient adsorption of heavy metal ions such as Cu2+, Cd2+ and Pb2+ from wastewater; ZHAO et al [14] prepared magnetic Fe3O4/graphene composites for effective uranium scavenging. BAI et al [15] studied the effects of reduced graphene oxide (rGO) content on the adsorption of rhodamine B and other dyes over composites comprised of rGO and manganese ferrite. Despite numerous magnetic composites were successfully used as adsorbents for the removal of pollutants in water systems, the application of them is limited due to factors such as complicated preparation, low adsorption capacity, poor separation efficiency, and harsh pH requirement. Endowed with unique properties, CoFe2O4@rGO composites have been widely used in catalysis, electromagnetic absorption, and lithium-ion batteries [16-18]. However, to the best of our awareness, there have been no reports on the synthesis of CoFe2O4@rGO composites using microwave hydrothermal method, as well as their use as adsorbents for the removal of uranium or other radioactive elements.
There are methods for the synthesis of nanomaterials including those that are based on hydrothermal, chemical precipitation, sol-gel, and ultrasonic approaches [19]. Most of these methods, however, have shortcomings such as easy aggregation of nanoparticles and long reaction time. In this paper, we reported the use of a microwave hydrothermal method for the synthesis of CoFe2O4@rGO. Compared to traditional hydrothermal synthesis, the deployment of microwave hydrothermal has additional advantages such as low energy consumption, rapid grain formation, and uniform particle size [20]. The morphology and structure of the magnetic CoFe2O4@rGO composite were characterized by XRD, Raman, FTIR, TEM, BET and VSM techniques. The adsorption of uranyl ions on CoFe2O4@rGO was studied, and the effect of experimental conditions such as time and pH on the adsorption was discussed. The excellent performance of CoFe2O4@rGO for uranium adsorption makes it potentially useful for the treatment of water that is contaminated with uranyl ions.
2 Experimental
2.1 Materials
Natural graphite (Alfar Aesar), uranium standard solution (100 μg/mL, Beijing Institute of Chemical Industry and Metallurgy), potassium permanganate (KMnO4), iron (III) chloride hexahydrate (FeCl3·6H2O), cobalt chloride hexahydrate (CoCl2·6H2O), sulfuric acid (H2SO4, 98%), hydrogen peroxide (H2O2, 30%), ammonia (NH3·H2O, 25%) and other chemicals were of analytical grade.
2.2 Preparation and characterization of CoFe2O4@rGO
Graphene oxide (GO) was prepared by a modified Hummers method [21]. The CoFe2O4@rGO nanocomposite was synthesized using a microwave-employed hydrothermal method. First 0.01 g of GO was dispersed in 10 mL of deionized water, and the mixture was ultrasonically treated for 2 h. Then 30 mL of FeCl3 and CoCl2 aqueous solution of specific concentration were slowly added to the GO solution at room temperature under N2 atmosphere. The pH of the reaction solution was adjusted to 10 using a 30% ammonia solution. The total volume of the solution was controlled at ca. 60 mL and stirring was continued for 30 min. The obtained precursor mixture was transferred to a polytetrafluoroethylene reactor with an effective volume of 100 mL, and placed in an MDS-6 microwave hydrothermal synthesizer. The heating rate was 10 °C/min, and the reaction was held at 160 °C for 30 min. After reaction, the mixture was naturally cooled to room temperature. The dark black solution was filtrated and the collected powder was washed with deionized water and ethanol for three times, and dried in an oven at 50 °C for 12 h to obtain a black powder sample, which is herein denoted as CoFe2O4@rGO. Pure CoFe2O4 and rGO were also synthesized following the procedures; for the former, there was no addition of GO whereas for the latter no addition of FeCl3 and CoCl2 aqueous solutions.
2.3 Characterization
The phase structure and purity of GO, rGO, CoFe2O4, and CoFe2O4@rGO samples were analyzed by D8 Advance X-ray powder diffractometer (XRD, Bruker D8, radiation source Cu Kα, wavelength 0.15406 nm, step size 0.04°, scanning range 20°-70°). The morphology of CoFe2O4@rGO was studied by TEM(H–7650B, 80 kV) and HRTEM (JEM-2010F, 120 kV). FTIR were carried out by Nicolet 6700 Fourier transform infrared spectrometer. Raman spectrum was analyzed by Raman spectrometer (Renishaw RM–1000, 514 nm, Ar-ion laser). Magnetic properties of synthetic samples were recorded on PPMS-9T at 298 K.
2.4 Adsorption experiment
40 mL of uranium solution of a particular concentration (10-100 mg/L) was added to an erlenmeyer flask, and the pH was adjusted to set value with 0.1 mol/L HCl or NaOH solution. After the addition of 8-60 mg of adsorbent into the flask, adsorption occurred with constant shaking at 298 K for a set time. After the adsorption process completed, the adsorbent was magnetically separated and the content of uranyl ions in the supernatant was analyzed by a MUA-type trace uranium measuring instrument. The adsorption capacity was calculated according to Eq. (1). The quasi-first-order kinetic and quasi-second-order kinetic models were used to evaluate the kinetic mechanism of the adsorption process, as shown in Eqs. (2) and (3), respectively [22]. As for the Langmuir and Freundlich isothermal adsorption equations, they are Eqs. (4) and (5) [23].
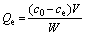 (1)
(1)
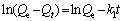 (2)
(2)
 (3)
(3)
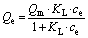 (4)
(4)
 (5)
(5)
where c0 and ce are the initial and adsorption equilibrium concentrations of uranium, respectively; W is the amount of adsorbent; V is the volume of the solution; Qe and Qt are the adsorption amounts at equilibrium and time t, respectively; k1 is the Lagergren rate constant; k2 is quasi-second-order kinetic adsorption rate constant; KL and KF are the Langmuir and Freundlich constants; n is the Freundlich adsorption index.
3 Results and discussion
3.1 Material characterization
Figure 1 shows the XRD patterns of GO, rGO and CoFe2O4@rGO. In the GO pattern, the diffraction peak at 10.8° is characteristic of graphite oxide. After microwave hydrothermal treatment, the peak at 10.8° has completely disappeared, and a weak broad peak at 24.5° attributable to reduced graphene oxide (002) appeared [24]. The XRD pattern of CoFe2O4@rGO shows a weak rGO peak at 24.6°, and other peaks at 30.2, 35.6, 43.2, 57.1, 62.6 and 74.5° appear which can be indexed to the (220), (311), (400), (511), (440) and (533) crystal planes of spinel-type CoFe2O4, respectively (JCPDS PDF card 22-1086) [25]. The average particle size of CoFe2O4 nanoparticles in the composite calculated using the Debye-Scherrer equation is about 14.0 nm. The XRD results indicate successful synthesis of the CoFe2O4@rGO complex.
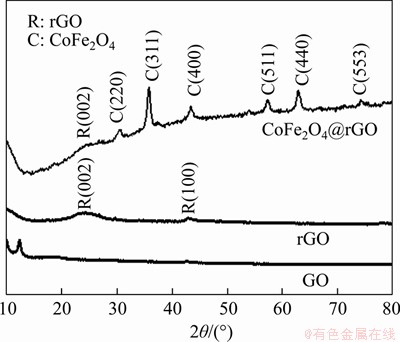
Figure 1 XRD patterns of GO, rGO and CoFe2O4@rGO
The FTIR spectra of GO and CoFe2O4@rGO are shown in Figure 2(a). According to literature, the infrared characteristic peaks of GO at 3416 and 1725 cm-1 are due to the stretching vibration of O—H and C=O, those at 1622, 1387, 1227 cm-1 are due to the stretching vibration of C—C, H2O, and O=C—O, while that at 1061 cm-1 is due to the stretching vibration of C—O—C [26]. Compared with GO, there is near complete disappearance of the characteristic peaks of oxygen-containing functional groups such as O—H, C=O, and C—O—C in the CoFe2O4@rGO case, implying that GO has been reduced to rGO. In addition, the strong absorption peak at 590 cm-1 is the vibrational peak of Fe—O, indicating successful loading of CoFe2O4 on rGO. Figure 2(b) shows the Raman spectra of GO and CoFe2O4@rGO, the G band at 1588 cm-1 is ascribed to the vibration of sp2 carbon atom, while the D band at 1346 cm-1 to the vibration of defects and disordered sp3 carbon atoms of graphene materials [27]. The D-band to G-band intensity ratio (ID/IG) of CoFe2O4@rGO (1.072) is higher than that of GO (0.931). It is possible that the reduction of GO and anchoring of CoFe2O4 nanoparticles on rGO cause an increase of disordered carbon atoms on the graphene surface [28].
The morphology of nanomaterials can be visually observed using the TEM and HRTEM techniques. Figure 3(a) shows the gauze-like morphology of GO curvy sheet. In Figure 3(b), one can see the clustering of CoFe2O4 nanoparticles which are 20-30 nm in size. Figures 3(c) and (d) are the TEM images of CoFe2O4@rGO with different magnifications, showing the CoFe2O4 nanoparticles are uniformly distributed on the graphene sheet (Figure 3(c)). The TEM image of higher magnification shows that the particle size of CoFe2O4 nanoparticles is about 13.4 nm (Figure 3(e)), which is consistent with the XRD result. The HRTEM image of CoFe2O4@rGO shows lattice fringes of 0.293 nm in spacing (Figure 3(f)), matching the (220) interplanar spacing of spinel-structure CoFe2O4 [29].
Figure 4(a) shows the magnetization hysteresis loops of CoFe2O4 and CoFe2O4@rGO collected at room-temperature. The CoFe2O4 nanoparticles and the CoFe2O4@rGO nanocomposite display superparamagnetism with coercivity close to zero. The saturation magnetizations of CoFe2O4 and CoFe2O4@rGO composite are 65.6 and 46.7 mA/(m2·g), respectively. The CoFe2O4@rGO composite has weaker magnetic properties than CoFe2O4, plausibly due to the coating of non-magnetic graphene on the surface of magnetic CoFe2O4. The CoFe2O4@rGO nanocomposite with a concentration of 2 mg/mL can be quickly separated from water under the action of an external magnetic field (inset of Figure 4(b)). Figure 5(b) shows the N2 adsorption-desorption and pore size distribution curves of CoFe2O4@rGO. The nanocomposite exhibits type-IV isotherm, and a H2-type hysteresis loop appears in the P/P* relative pressure range of 0.65-0.95, which indicate existence of mesoporous structure. The specific surface area and pore volume of CoFe2O4@rGO are 119.76 m2/g and 0.1822 cm3/g, respectively. The distribution of pore size is mainly at 4.76 nm as calculated by BJH model (inset of Figure 5(b)). The large specific surface area of CoFe2O4@rGO indicates high adsorption capacity and abundant adsorption sites, thereby facilitating effective adsorption.
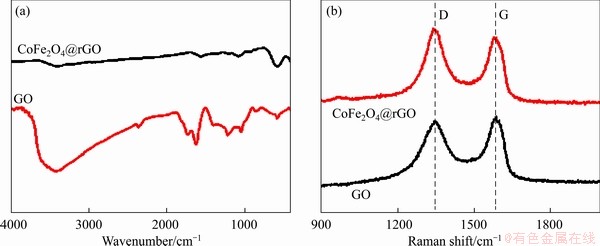
Figure 2 FTIR spectra (a) and Raman spectra (b) of GO and CoFe2O4@rGO
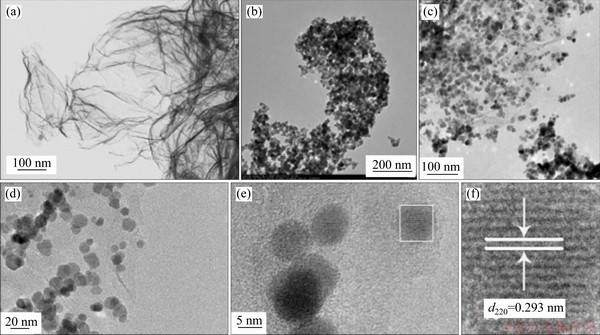
Figure 3 TEM images of (a) GO, (b) CoFe2O4, (c, d) CoFe2O4@rGO; (e)TEM and (f) HRTEM image of CoFe2O4@rGO
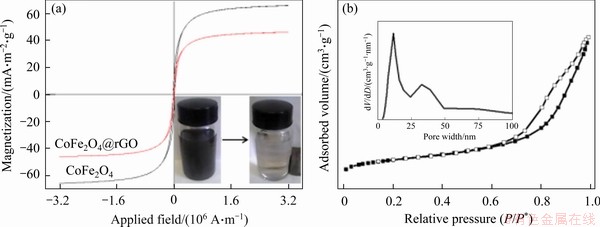
Figure 4 Magnetization curves (inset are pictures illustrating CoFe2O4@rGO magnetic separation) (a) and nitrogen adsorption–desorption isotherm curves of CoFe2O4@rGO (b)
3.2 Adsorption performance
The effects of solution pH (2-11) on the removal of uranium (initial concentration 100 mg/L) using CoFe2O4, rGO and CoFe2O4@rGO are illustrated in Figure 5. It can be seen that rGO exhibits the lowest adsorption performance whereas CoFe2O4@rGO shows the highest. The CoFe2O4@rGO composite has a uranium adsorption capacity of 127.6 mg/g, which is 1.31 and 2.43 times that of rGO and pure CoFe2O4, respectively. The capacity of CoFe2O4@rGO for uranium adsorption is greatly affected by the pH value of the solution. When pH increases from 2.0 to 4.0, there is gradual increase of adsorption capacity. Within the pH range of 4.0-6.0, the adsorption capacity remains roughly unchanged. When pH is above 6.0, the adsorption capacity progressively decreases. It is considered that at pH<4, U(VI) exists as UO22+ in the solution, and the lower uranium adsorption capacity is due to the competition between H+ and UO22+ for adsorption sites [27]. When pH is between 4 and 6, (UO2)3(OH)5+ dominates [30], and adsorption is significantly improved due to electrostatic interaction between positively charged UO2)3(OH)5+ and negatively charged CoFe2O4@rGO surface. When pH is larger than 8.0, UO2(CO3)22- and UO2(CO3)34- are the main uranium species, and these anions have low affinity with negatively charged CoFe2O4@rGO composite, leading to rapid decrease of adsorption capacity. The results show that the optimal pH for uranium adsorption on CoFe2O4@rGO is 5.0.
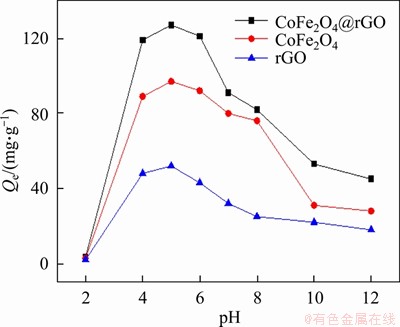
Figure 5 Influence of pH value on adsorption efficiency of as-synthesized materials (m/V=0.5 g/L, shaking time: 180 min)
The effects of solid-liquid ratio and initial uranium concentration on the adsorption performance were further analyzed. The results of solid-liquid ratio are shown in Figure 6(a) (initial uranium concentration=40 mg/L), with the increase of solid-liquid ratio from 0.2 to 0.5 g/L, the uranium adsorption rate increases from 58.4% to 92.2%. When the solid-liquid ratio is further increased to 1.0 g/L, the uranium adsorption rate remains almost unchanged, but adsorption capacity continues to decrease. The possible reason is that all kinds of energy adsorption sites on CoFe2O4@rGO surface can be completely exposed for adsorption at low solid-liquid ratio, and the surface saturation is faster, so it shows higher adsorption capacity. However, at high solid-liquid ratio, most of the sites with lower energy are preferentially used for adsorption, resulting in the decrease of binding properties of the higher energy sites. In addition, the higher solid-liquid ratio will increase the probability of collision between adsorbent particles, thus resulting in aggregation. Both of them will reduce the adsorption capacity. Figure 6(b) shows the effect of initial uranium concentration on adsorption performance (solid-liquid ratio=0.5 g/L). With the increase or decrease of initial concentration, the adsorption capacity increases and the adsorption rate decreases. Therefore, the optimum solid-liquid ratio and initial are 0.5 g/L and 100 mg/L, respectively.

Figure 6 Effect of solid-liquid ratio (a) and initial uranium concentration (b) on adsorption efficiency (pH=5, shaking time: 180 min)
Adsorption kinetics of an adsorption process can reflect the adsorption efficiency of an adsorbent. The effect of adsorption time on uranium adsorption over CoFe2O4@rGO is depicted in Figure 7(a). It can be seen that there is rapid adsorption during the first 90 min, and then adsorption rate slows down before reaching equilibrium at 150 min. Therefore, to ensure adsorption equilibrium, the adsorption time was set to 150 min in subsequent experiments. The results show that the maximum adsorption capacity of CoFe2O4@rGO is 135.4 mg/g.
The quasi-first-order kinetic equation and quasi-second-order kinetic equation were fitted to the experimental data of uranium adsorption on CoFe2O4@rGO at 298 K. The results are shown in Figures 7(b) and (c) and the related kinetic data are listed in Table 1. It is observed that the theoretical adsorption capacity calculated using the quasi-first-order kinetic model is inconsistent with the experimental data, and the correlation coefficient is relatively low (0.954), indicating that the adsorption of uranium by CoFe2O4@rGO does not conform to the quasi-first-order kinetic model. The theoretical saturated adsorption Qe (cal) calculated according to the quasi-second-order kinetic equation is more in line with the experimental Qe (exp), and the correlation coefficient is close to 1. Therefore, the adsorption process of uranium on CoFe2O4@rGO is better described by the quasi-second-order kinetic model.

Figure 7 Uranium sorption of CoFe2O4@rGO versus time (a); pseudo-first-order (b) and pseudo-second-order adsorption fit plot (c) for uranium sorption (pH=5, m/V=0.5 g/L)
To further study the adsorption process, Langmuir and Freundlich isothermal adsorption models were used to analyze the adsorption equilibrium data. The results are shown in Figure 8 and the adsorption parameters are compiled in Table 2. The correlation coefficient (R2) of Langmuir isotherm fitting for rGO and CoFe2O4@rGO is 0.998 and 0.998, respectively, which is higher than that of Freundlich isotherm fitting (0.848 and 0.798). It is clear that the adsorption processes of uranyl ions are more consistent with the Langmuir model, indicating uniform monolayer adsorption of uranyl ions on rGO and CoFe2O4@rGO. According to Langmuir adsorption isotherm calculation, the maximum capacity (Qm) of CoFe2O4@rGO for uranium sorption at 25 °C is 160.5 mg/g. The fitting result of Freundrich isothermal adsorption model suggests that the n value is less than 1, and when 0
There are many kinds of metal ions in the actual uranium-containing wastewater, and these ions could affect the uranium adsorption performance of CoFe2O4@rGO, because they compete with uranyl ions for adsorption sites and obstruct the adsorption of uranium. Figure 9(a) shows the adsorption results of CoFe2O4@rGO in actual uranium-containing wastewater (initial pH value 4-6, cNa: 0.3-10.0 mg/L, cK: 0.3-10.0 mg/L, cSi≤2.5 mg/L, cCa≤5.00 mg/L, cFe≤0.34 mg/L, cMg≤1.38 mg/L and a small amount of Al, B, Be, Cu, Mo, Ni, etc.). It can be seen that the adsorption capacity of CoFe2O4@rGO in uranium-containing seawater is lower than that in a solution just with the presence of uranium. This may be due to the competitive adsorption of other metal ions and/or the pH of the uranium-containing seawater solution which is deviated from the optimal pH for uranium adsorption. The effect of the coexisting of Na+, K+, Ca2+ or Mg2+ cations on uranium adsorption performance was also investigated. The results show the co-presence of any of the four cations has little effect on the uranium adsorption performance of CoFe2O4@rGO (Figure 9(b)). Among them, Na+ and K+ have insignificant effect on the uranium adsorption performance of CoFe2O4@rGO. The effect of Ca2+ and Mg2+ of higher valence is marginally more significant. The above results indicate that CoFe2O4@rGO has good selectivity towards uranium ions.
Table 1 Pseudo-first-order and pseudo-second-order model parameters for CoFe2O4@rGO

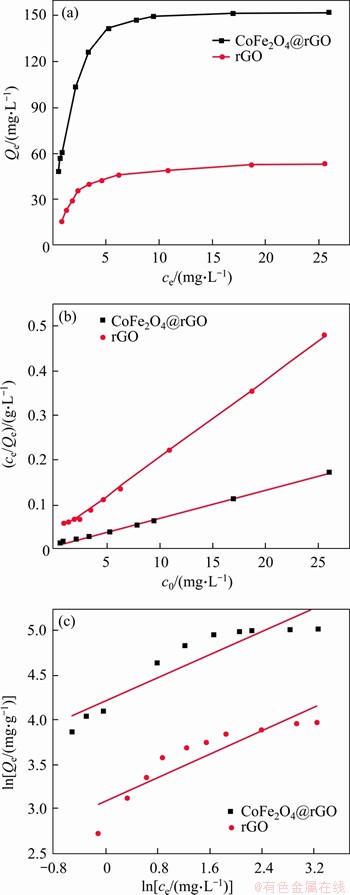
Figure 8 Adsorption isotherm (a), Langmuir model (b), and Freundlich model (c) of CoFe2O4@rGO and rGO
The recycling of the adsorbent is an important indicator of its practical application value. The adsorption-desorption cycle experiments of uranyl solution by CoFe2O4@rGO nanocomposite was carried out. In the desorption experiment, the adsorbent after the end of the adsorption was stirred with a desorption solution of 0.1 mol/L of NaOH, NaHCO3 or Na2CO3 for 60 min, and the regenerated adsorbent was obtained by drying after applying magnetic separation. Figure 10(a) shows that the desorption rates of uranyl ion desorbed from the adsorbent were 61.2%, 92.3% and 84.5%, indicating that the desorption effect is the best when using NaHCO3 desorption solution and used in subsequent adsorption-desorption cycle experiments. Figure 10(b) shows that after five adsorption-desorption cycles, the adsorption capacity was slightly reduced from 126 to 108.5 mg/g, and still reached about 98% of the first adsorption capacity. It can be seen that CoFe2O4@rGO has better cycle performance and has potential for practical applications.
4 Conclusions
Magnetic CoFe2O4@rGO composite was successfully prepared using microwave hydrothermal method. The structure and morphology of GO, rGO, CoFe2O4, and CoFe2O4@rGO were characterized by XRD, SEM, TEM, VSM, etc., and tested for uranium adsorption in aqueous media. The effects of pH, adsorption time and initial concentration of uranium on the adsorption behaviour of rGO, CoFe2O4, and CoFe2O4@rGO were investigated. The results show that CoFe2O4@rGO performs well for uranium adsorption, and pH has a significant effect on adsorption capacity. At a solution pH of 5, the uranium adsorption capacity of CoFe2O4@rGO is 127.6 mg/g, which is 1.31 and 2.43 times that of rGO and pure CoFe2O4, respectively. The uranium kinetic and adsorption isotherm data of CoFe2O4@rGO are more closely fitted to the quasi-second-order kinetic equation of Langmuir isotherm model. It is envisaged that the magnetic CoFe2O4@rGO composite is a promising material for the treatment of uranium-containing wastewater.
Table 2 Adsorption parameters for Langmuir and Freundlich isotherm models

Figure 9 Uranium adsorption on CoFe2O4@rGO in actual wastewater (a) and effect of coexisting ions on uranium adsorption performance (b)

Figure 10 Desorption rate of different desorption agents (a), adsorption capacity for five cycles (b)
Contributors
The overarching research goals were developed by WU Shui-sheng and YI Bing. LAN Dong-hui and ZHANG Xiao-wen carried out mechanical tests. HUANG Yi and DENG Xing-hong analyzed the data. WU Shui-sheng and AU Chak-tong wrote the paper. All authors replied to reviewers’comments and revised the final version.
Conflict of interest
All authors declare that they have no conflict of interest.
References
[1] YANG Ai-li, YANG Peng, HUANG C P. Effect of Mg(II) on the removal of uranium from low radioactive wastewater by flocculation using polyacrylamide [J]. Journal of Hazardous, Toxic, and Radioactive Waste, 2017, 21(4): 04017006. DOI: 10.1061/(asce)hz.2153-5515.0000359.
[2] ZHANG C, DODGE C J, MALHOTRA S V, FRANCIS A J. Bioreduction and precipitation of uranium in ionic liquid aqueous solution by Clostridium sp [J]. Bioresource Technology, 2013, 136: 752-756. DOI: 10.1016/j.biortech. 2013.03.085.
[3] SHEN Jun-jie, SCHAFER A. Removal of fluoride and uranium by nanofiltration and reverse osmosis: A review [J]. Chemosphere, 2014, 117: 679-691. DOI: 10.1016/ j.chemosphere.2014.09.090.
[4] SREENIVAS T, RAJAN K C. Studies on the separation of dissolved uranium from alkaline carbonate leach slurries by resin-in-pulp process [J]. Separation and Purification Technology, 2013, 112: 54-60. DOI: 10.1016/j.seppur. 2013.03.050.
[5] LINGAMDINNE L P, CHOI Y L, KIM I S, YANG J K, KODURU J R, CHANG Y Y. Preparation and characterization of porous reduced graphene oxide based inverse spinel nickel ferrite nanocomposite for adsorption removal of radionuclides [J]. Journal of Hazardous Materials, 2017, 326: 145-156. DOI: 10.1016/j.jhazmat.2016.12.035.
[6] LI J, WANG X, ZHAO G, CHEN C, CHAI Z, ALSAEDI A, HAYAT T, WANG X. Metal-organic framework-based materials: Superior adsorbents for the capture of toxic and radioactive metal ions [J]. Chemical Society Reviews, 2018, 47(7): 2322-2356. DOI: 10.1039/c7cs00543a.
[7] TOBILKO V, SPASONOVA L, KOVALCHUK I, KORNILOVYCH B, KHOLODKO Y. Adsorption of uranium (VI) from aqueous solutions by amino-functionalized clay minerals [J]. Colloids and Interfaces, 2019, 3(1): 41. DOI: 10.3390/colloids3010041.
[8] ANIRUDHAN T S, JALAJAMONY S. Ethyl thiosemicarbazide intercalated organophilic calcined hydrotalcite as a potential sorbent for the removal of uranium(VI) and thorium(IV) ions from aqueous solutions [J]. Journal of Environmental Sciences, 2013, 25(4): 717-725. DOI: 10.1016/S1001-0742(12)60064-3.
[9] GU Peng-cheng, ZHANG Sai, LI Xing, WANG Xiang-xue, WEN Tao, JEHAN R, ALSAEDI A, HAYAT T, WANG Xiang-ke. Recent advances in layered double hydroxide-based nanomaterials for the removal of radionuclides from aqueous solution [J]. Environmental Pollution, 2018, 240: 493-505. DOI: 10.1016/j.envpol.2018.04.136.
[10] CHOUYYOK W, WARNER C L, MACKIE K E, WARNER M G, GILL G A, ADDLEMAN R S. Nanostructured metal oxide sorbents for the collection and recovery of uranium from seawater [J]. Industrial & Engineering Chemistry Research, 2016, 55(15): 4195-4207. DOI: 10.1021/acs.iecr.5b03650.
[11] WEN Zhen-qian, YAO Yi-xuan, NIU Yu-qing, ZHOU Gen-mao, XU Guo-long, ZHONG Hong. Adsorption mechanism of weakly basic anion exchange resin for uranium in acidic leaching solution containing uranium [J]. Journal of Central South University (Science and Technology), 2016, 47(6): 1867-1871. (in Chinese)
[12] ALI I, BASHEER A A, MBIANDA X Y, BURAKOV A, GALUNIN E, BURAKOVA I, MKRTCHYAN E, TKACHEV A, GRACHEV V. Graphene based adsorbents for remediation of noxious pollutants from wastewater [J]. Environment International, 2019, 127: 160-180. DOI: 10.1016/j.envint. 2019.03.029.
[13] WU Zhong-shuai, WANG Da-wei, REN Wen-cai, ZHAO Jin-ping, ZHOU Guang-min, LI Feng, CHENG Hui-ming. Anchoring hydrous RuO2 on graphene sheets for high-performance electrochemical capacitors [J]. Advanced Functional Materials, 2010, 20(20): 3595-3602. DOI: 10.1002/adfm.201001054.
[14] ZHAO Dong-lin, ZHU Hong-yu, WU Chang-nian, FENG Shao-jie, ALSAEDI A, HAYAT T, CHEN Chang-lun. Facile synthesis of magnetic Fe3O4/graphene composites for enhanced U(VI) sorption [J]. Applied Surface Science, 2018, 444: 691-698. DOI: 10.1016/j.apsusc.2018.03.121.
[15] BAI Song, SHEN Xiao-ping, ZHONG Xin, LIU Yang, ZHU Guo-xing, XU Xiang, CHEN Kang-min. One-pot solvothermal preparation of magnetic reduced graphene oxide-ferrite hybrids for organic dye removal [J]. Carbon, 2012, 50(6): 2337-2346. DOI: 10.1016/j.carbon.2012.01.057.
[16] CHEN Teng, DU Ping, JIANG Wei, LIU Jie, HAO Ga-zi, GAO Han, XIAO Lei, KE Xiang, ZHAO Feng-qi. A facile one-pot solvothermal synthesis of CoFe2O4/RGO and its excellent catalytic activity on thermal decomposition of ammonium perchlorate [J]. RSC Advances, 2016, 6(87): 83838-83847. DOI: 10.1039/c6ra16448j.
[17] ZHANG Kun, LI Jun-jian, WU Fan, SUN Meng-xiao, XIA Yi-lu, XIE A-ming. Sandwich CoFe2O4/RGO/CoFe2O4 nanostructures for high-performance electromagnetic absorption [J]. ACS Applied Nano Materials, 2019, 2(1): 315-324. DOI: 10.1021/acsanm.8b01927.
[18] ZHU Yan-fang, LV X, ZHANG Li-li, GUO Xiao-dong, LIU Dai-jun, CHEN Jian-jun, JI Jun-yi. Liquid-solid-solution assembly of CoFe2O4/graphene nanocomposite as a high-performance lithium-ion battery anode [J]. Electrochimica Acta, 2016, 215: 247-252. DOI: 10.1016/j.electacta. 2016.08.057.
[19] SHARMA N, OJHA H, BHARADWAJ A, PATHAK D P, SHARMA R K. Preparation and catalytic applications of nanomaterials: A review [J]. RSC Advances, 2015, 5(66): 53381-53403. DOI: 10.1039/C5RA06778B.
[20] CORRADI A B, BONDIOLI F, FOCHER B, FERRARI A M, GRIPPO C, MARIANI E, VILLA C. Conventional and microwave-hydrothermal synthesis of TiO2 nanopowders [J]. Journal of the American Ceramic Society, 2005, 88(9): 2639-2641. DOI: 10.1111/j.1551-2916.2005.00474.x.
[21] MA Jun, LIU Chang-hua, LI Rui, WANG Jia. Properties and structural characterization of oxide starch/chitosan/graphene oxide biodegradable nanocomposites [J]. Journal of Applied Polymer Science, 2012, 123(5): 2933-2944. DOI: 10.1002/ app.34901.
[22] WEN Xiao-feng, DU Chun-yan, ZENG Guang-ming, HUANG Dan-lian, ZHANG Jin-fan, YIN Ling-shi, TAN Shi-yang, HUANG Lu, CHEN Hong. A novel biosorbent prepared by immobilized Bacillus licheniformis for lead removal from wastewater [J]. Chemosphere, 2018, 200: 173-179. DOI: 10.1016/j.chemosphere.2018.02.078.
[23] LIU Wen, ZHAO Xiao, WANG Ting, ZHAO Dong-ye, NI Jin-ren. Adsorption of U(VI) by multilayer titanate nanotubes: Effects of inorganic cations, carbonate and natural organic matter [J]. Chemical Engineering Journal, 2016, 286: 427-435. DOI: 10.1016/j.cej.2015.10.094.
[24] FATHY M, GOMAA A, TAHER F A, EL-FASS M M, KASHYOUT A E H B. Optimizing the preparation parameters of GO and rGO for large-scale production [J]. Journal of Materials Science, 2016, 51(12): 5664-5675. DOI: 10.1007/ s10853-016-9869-8.
[25] GABAL M A, AL-JUAID A A, EL-RASHED S, HUSSEIN M A. Synthesis and characterization of nano-sized CoFe2O4 via facile methods: A comparative study [J]. Materials Research Bulletin, 2017, 89: 68-78. DOI: 10.1016/j.materresbull. 2016.12.048.
[26] MERMOUX M, CHABRE Y, ROUSSEAU A. FTIR and 13C NMR study of graphite oxide [J]. Carbon, 1991, 29(3): 469-474. DOI: 10.1016/0008-6223(91)90216-6.
[27] CHEN Shui-ping, HONG Jian-xun, YANG Hong-xiao, YANG Ji-zhen. Adsorption of uranium (VI) from aqueous solution using a novel graphene oxide-activated carbon felt composite [J]. Journal of Environmental Radioactivity, 2013, 126: 253-258. DOI: 10.1016/j.jenvrad.2013.09.002.
[28] SUN Yu-bing, DING Cong-cong, CHENG Wen-cai, WANG Xiang-ke. Simultaneous adsorption and reduction of U(VI) on reduced graphene oxide-supported nanoscale zerovalent iron [J]. Journal of Hazardous Materials, 2014, 280: 399-408. DOI: 10.1016/j.jhazmat.2014.08.023.
[29] YIN Wen-zhu, HAO Shuo, CAO Hua-qiang. Solvothermal synthesis of magnetic CoFe2O4/rGO nanocomposites for highly efficient dye removal in wastewater [J]. RSC Advances, 2017, 7(7): 4062-4069. DOI: 10.1039/C6RA26948F.
[30] SUN Yu-bing, YANG Shi-tong, SHENG Guo-dong, GUO Zhi-qiang, WANG Xiang-ke. The removal of U(VI) from aqueous solution by oxidized multiwalled carbon nanotubes [J]. Journal of Environmental Radioactivity, 2012, 105: 40-47. DOI: 10.1016/j.jenvrad.2011.10.009.
(Edited by HE Yun-bin)
中文导读
CoFe2O4@rGO 纳米复合材料的微波水热合成、表征及其吸附性能
摘要:通过微波水热法合成磁性CoFe2O4@rGO 纳米复合材料。XRD、Raman、TEM/HRTEM、FTIR、BET和VSM表征结果表明,尺寸约为13 nm的尖晶石型CoFe2O4纳米粒子分散锚定在石墨烯片上,其饱和磁化强度为46.7 mA/(m2·g),满足磁分离要求。研究了不同pH值、初始浓度等条件对CoFe2O4@rGO铀吸附容量的影响,确定了铀在水中的吸附行为并拟合了吸附动力学方程。结果表明在pH=5时,CoFe2O4@rGO 纳米复合材料吸附铀的能力为127.6 mg/g,分别是rGO和纯CoFe2O4的1.31和2.43倍。吸附过程符合Langmuir和准二级动力学模型。CoFe2O4@rGO纳米复合材料优良的吸附性能使其在处理铀污染水方面具有潜在的用途。
关键词:CoFe2O4;石墨烯;铀;吸附性能
Foundation item: Project(19B126) supported by the Scientific Research Fund of Hunan Provincial Education Department, China; Project(21772035) supported by the National Natural Science Foundation of China; Projects(2018JJ3099, 2019JJ40058) supported by the Provincial Natural Science Foundation of Hunan, China; Project supported by the Innovation and Entrepreneurship Training Program of Hunan Institute of Engineering, China
Received date: 2020-05-09; Accepted date: 2020-12-01
Corresponding author: YI Bing, PhD, Professor; Tel: +86-13203218660; E-mail: bingyi2004@126.com; ORCID: https://orcid.org/0000-0002-8513-7051

