Red-blood-cell-like nitrogen-doped porous carbon as an efficient metal-free catalyst for oxygen reduction reaction
来源期刊:中南大学学报(英文版)2019年第6期
论文作者:唐有根 彭志光 王莉萍 田敬 李静莎 曾宪光 黄小兵 王海燕
文章页码:1458 - 1468
Key words:oxygen reduction reaction; nitrogen-doped carbon; porous structure; red-blood-cell-like morphology
Abstract: A red-blood-cell-like nitrogen-doped porous carbon catalyst with a high nitrogen content (9.81%) and specific surface area (631.46 m2/g) was prepared by using melamine cyanuric acid and glucose as sacrificial template and carbon source, respectively. This catalyst has a comparable onset potential and a higher diffusion-limiting current density than the commercial 20 wt% Pt/C catalyst in alkaline electrolyte. The oxygen reduction reaction mechanism catalyzed by this catalyst is mainly through a 4e pathway process. The excellent catalytic activity could origin from the synergistic effect of the in-situ doped nitrogen (up to 9.81%) and three-dimensional (3D) porous network structure with high specific surface area, which is conducive to the exposure of more active sites. It is interesting to note that the catalytic activity of oxygen reduction strongly depends on the proportion of graphic N rather than the total N content.
Cite this article as: WANG Li-ping, TIAN Jing, LI Jing-sha, ZENG Xian-guang, PENG Zhi-guang, HUANG Xiao-bing, TANG You-gen, WANG Hai-yan. Red-blood-cell-like nitrogen-doped porous carbon as an efficient metal-free catalyst for oxygen reduction reaction [J]. Journal of Central South University, 2019, 26(6): 1458-1468. DOI: https://doi.org/10.1007/s11771-019-4102-y.

ARTICLE
J. Cent. South Univ. (2019) 26: 1458-1468
DOI: https://doi.org/10.1007/s11771-019-4102-y

WANG Li-ping(王莉萍)1, TIAN Jing(田敬)1, LI Jing-sha(李静莎)1,
ZENG Xian-guang(曾宪光)2, PENG Zhi-guang(彭志光)1, HUANG Xiao-bing(黄小兵)3,
TANG You-gen(唐有根)1, WANG Hai-yan(王海燕)1
1. Hunan Provincial Key Laboratory of Chemical Power Sources, Hunan Provincial Key Laboratory of
Efficient and Clean Utilization of Manganese Resources, College of Chemistry and Chemical Engineering,Central South University, Changsha 410083, China;
2. Material Corrosion and Protection Key Laboratory of Sichuan Province, Zigong 643000, China;
3. College of Chemistry and Materials Engineering, Hunan University of Arts and Science,Changde 415000, China
 Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2019
Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2019
Abstract: A red-blood-cell-like nitrogen-doped porous carbon catalyst with a high nitrogen content (9.81%) and specific surface area (631.46 m2/g) was prepared by using melamine cyanuric acid and glucose as sacrificial template and carbon source, respectively. This catalyst has a comparable onset potential and a higher diffusion-limiting current density than the commercial 20 wt% Pt/C catalyst in alkaline electrolyte. The oxygen reduction reaction mechanism catalyzed by this catalyst is mainly through a 4e pathway process. The excellent catalytic activity could origin from the synergistic effect of the in-situ doped nitrogen (up to 9.81%) and three-dimensional (3D) porous network structure with high specific surface area, which is conducive to the exposure of more active sites. It is interesting to note that the catalytic activity of oxygen reduction strongly depends on the proportion of graphic N rather than the total N content.
Key words: oxygen reduction reaction; nitrogen-doped carbon; porous structure; red-blood-cell-like morphology
Cite this article as: WANG Li-ping, TIAN Jing, LI Jing-sha, ZENG Xian-guang, PENG Zhi-guang, HUANG Xiao-bing, TANG You-gen, WANG Hai-yan. Red-blood-cell-like nitrogen-doped porous carbon as an efficient metal-free catalyst for oxygen reduction reaction [J]. Journal of Central South University, 2019, 26(6): 1458-1468. DOI: https://doi.org/10.1007/s11771-019-4102-y.
1 Introduction
For the sluggish kinetics of oxygen reduction reaction (ORR) at cathode, developing an efficient electrocatalyst for ORR has been considered as the key whether the commercial application of metal- air batteries can be achieved. Till now, Pt-based materials have been as the state of the art electrocatalysts for ORR and a standard for evaluating the performance of electrocatalysts. Nevertheless, the scarcity and high cost of platinum limit its commercial applications [1-6]. Hence, extensive effort has been devoted to developing highly efficient but low-cost electrocatalysts for ORR, mainly including non-precious metal WANG Li-ping and TIAN Jing contributed equally to this work.materials and even metal-free materials. Recently, metal-free catalysts have received a lot of attention for their lower cost and pollution than those metal- containing catalysts [2, 3]. Many studies have reported that the incorporation of heteroatoms (such as, N, P, S, and B) is able to effectively adjust the electronic state of the carbon skeleton, which could greatly influence the oxygen adsorption, the information of active sites and the selectivity of reaction pathway of electrocatalysts [6-13]. Among all metal-free heteroatom-doped carbon materials, N-doped carbon (N-C) materials are believed to be the most promising metal-free electrocatalysts because of the low cost, easy preparation, low pollution, outstanding activity and high stability [6, 8]. The doping of electron-rich nitrogen atoms makes the adjacent C atoms with a positive charge, which could weaken the bind energy of O-O band and facilitate the absorption of oxygen.
Recently, N-C catalysts with various morphologies (e.g., nanotubes, mesoporous spheres, and nanocages) have been investigated as novel electrocatalysts towards ORR. Especially, the morphologies featuring 3D mesoporous network generally exhibit outstanding electrocatalytic activity since they can expose more catalytic active sites and facilitate the absorption-diffusion of oxygen and the transport of electron [13-19]. So far, the preparation methods of N-C catalysts can mainly be classified as two categories. One is post-doping, namely the post-heating of the graphitic carbon together with N-containing composites. The nitrogen content of the catalysts synthesized by this method is usually too low to form enough active sites. The other is in-situ N atoms doping into the C skeleton during the formation process of graphitic carbon. This method usually could obtain a high nitrogen content. However, most materials by this method are too complex to receive a mass production [19-23]. Generally, these catalysts with 3D mesoporous network structure and high nitrogen content often could be obtained by using the soft or hard templates, such as mesoporous silica template and mesoporous alumina template, which can not only receive a high surface area by forming porous structure but also succeed in reserving the N species of the precursors to gain a high nitrogen content. Whereas, most of the template methods reported previously, such as, mesoporous silica and alumina, are hard to remove completely [13, 17, 19]. Though many kinds of N-C materials possessing excellent activity for ORR have been reported, the catalytic mechanism and the real catalytic sites have not been fully elaborated yet [23-26].
In this work, a kind of red-blood-cell-like N-C catalyst with 3D porous structure and high nitrogen content (9.81%) is synthesized by using a facile two-step method [22]. As we know, red blood cells are able to maximize the absorption of the surrounding oxygen probably owing to their special shape, which is like a depression in the middle of the small round cakes and makes them have a higher surface area than that of the sphere morphology. The unique red-blood-cell-like morphology of N-C catalysts is also in favor of the absorption-diffusion of oxygen and the electron transport during the ORR process because of its 3D mesoporous network structure and high surface area (631.46 m2/g) [22, 27]. Based on a series of optimum experiments, the optimized N-C catalyst exhibits high-performance electrocatalytic activity, approaching to those of commercial 20% Pt/C under the same test conditions. The influence of pyrolysis temperature on the ORR performance was investigated in details and the intrinsic reasons for the different N species were demonstrated.
2 Experimental
2.1 Synthesis of NC catalysts
All reagents were used as purchased without further purification. In a typical preparation process, 0.5 g melamine and 0.51 g cyanuric acid were completely dissolved in a certain amount of moderate dimethyl sulfoxide (DMSO), respectively, and then both solutions were mixed together and continuously stirred until the reactants reacted completely to form MCA templates. The suspension was filtrated to separate the MCA product, which was washed with ethanol three times and dried in oven at 70 °C. 800 mg MCA and 100 mg glucose were mixed and grounded in an agate mortar to get a power mixture, then it was pyrolyzed as follows. Firstly, the mixture was heated at 150 °C for 1 h with a heating rate of 5 °C/min in Ar atmosphere so that the glucose was completely melted and filled in MCA template. And then, the pyrolysis temperature was increased to 800 °C for 3 h with a heating rate of 5 °C/min under Ar. The as-prepared sample was denoted as N-C-800.
For comparison, a series of N-C controlled samples were synthesized by the same process except the pyrolysis temperature or the mass ratio of MCA and glucose (N/C). Furthermore, to emphasize the important role of MCA as self-template and nitrogen sources, glucose was also pyrolyzed at 800 °C for 3 h and the obtained sample was designed as Glucose-800 [22].
2.2 Characterization
The X-ray diffraction (XRD) patterns of materials were recorded on Dandong Haoyuan DX-2700 diffractometer with Cu Kα1 radiation source. The Raman spectra were received by using a Jasco Laser Raman Spectrophotometer NRS-3000 Series. The scanning electron microscopy (SEM) images of materials were performed on a Nova NanoSEM 230 SEM. The transmission electron microscopy (TEM), high- resolution TEM (HRTEM) images, and elemental mapping of materials were obtained by using a FEI Tecnai G2 F20 S-TWIX TEM. The X-ray photoelectron spectroscopy (XPS) data of materials were obtained from a K-Alpha1063 spectrometer. The specific surface area data of materials were achieved on a Brunauer-Emmett-Teller (BET) equation (SSA-4200) by N2 adsorption isotherms.
2.3 Electrochemical tests
All electrochemical tests of the samples were performed by using a CHI760e electrochemical workstation and a Pine-Instrument with a three- electrode system in 0.1 mol/L KOH electrolyte. The three-electrode system consists of counter electrode (Pt wire), reference electrode (saturated calomel electrode, SCE) and working electrode. The working electrode was prepared by loading 10 μL catalyst ink onto a 5.61 mm diameter glassy carbon rotating disk electrode (Pine Instrument Company, USA) encircled by Pt ring. The preparation method of catalyst ink is as follows: 6 mg catalyst powder and 950 μL ethanol were mixed for half an hour of ultrasonic processing and then 50 μL Nafion was added into the above suspension for another 30 min of ultrasound to form a homogeneous ink. All potentials measured was converted into the standard of reversible hydrogen electrode (RHE). The linear sweep voltammograms (LSV) of materials were carried out with a scanning rate of 10 mV/s from 400 to 1600 r/min in an oxygen saturated 0.1 mol/L KOH electrolyte. The cyclic voltammograms (CVs) were also conducted in the potential range from 0 to 1.2 V under the same conditions.
The hydrogen peroxide yield (YH2O2) and electron transfer number (n) during ORR process were calculated based on rotating ring-disk electrode (RRDE) measurement. The calculation formulas are as follows [28-30]:
 (1)
(1)
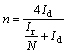 (2)
(2)
where Id and Ir are disk and ring current density, respectively; N is the current collection efficiency of Pt ring (N=0.37), which is a constant.
3 Results and discussion
SEM images of MCA template and N-C materials at different magnifications are shown in Figure 1. As seen, the morphology of MCA template (Figure 1(a)) shows a uniform rose-like structure, which is assembled by a pile of nanosheets. It is interesting to note that the N-C-800 catalyst (Figures 1(b) and (c)) exhibits a uniform red-blood-cell-like morphology, which is linked by three-dimensional (3D) porous network. The fluffy and abundant porous structure of this sample is in favor of the exposure of active sites and can facilitate the adsorption and diffusion of oxygen and the transport of electron. The SEM images of N-C-900 are also given in Figure 1(d). As can be seen, the red-blood-cell like morphology seems to be destroyed to form a flower-like structure when the pyrolysis temperature is increased to 900 °C. Note that the yield of N-C-900 is obviously decreased, therefore, we choose the sample N-C-800 for further investigation. Figure 1(e) shows a smooth large bulk morphology for Glucose-800 sample without the use of MCA template, revealing the importance of this template in morphology forming. Furthermore, we have also tried to modify the N-C material with trace Fe to increase its electrocatalytic activity, but this approach was negated by the serious morphology destroy (Figure 1(f)) and the inferior ORR activity (Figure 7(b)).
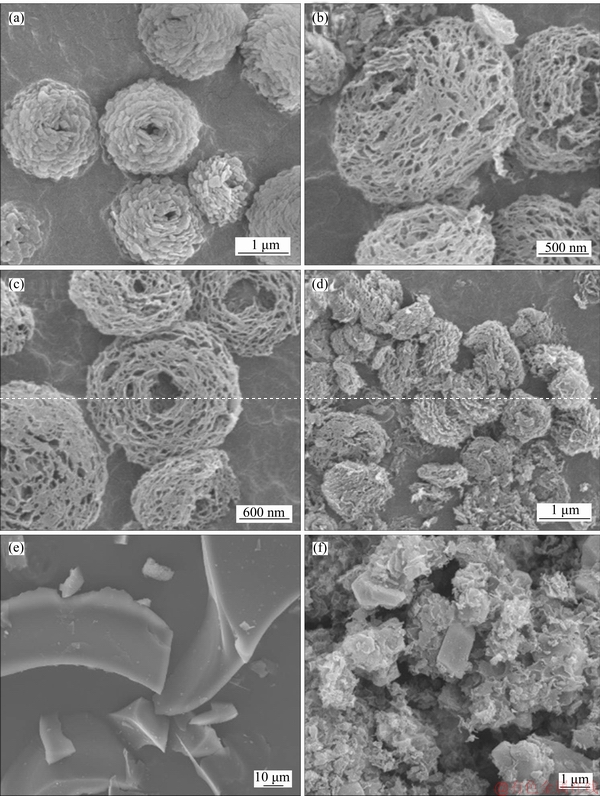
Figure 1 SEM images of MCA template (a), N-C-800 catalyst (b-c), N-C-900 catalyst (d), Glucose-800 catalyst (e), and Fe-N-C-800 catalyst (f) under different magnifications
To further understand the microstructure of N-C-800 catalyst, TEM and HRTEM images of N-C-800 catalyst are obtained. The TEM image (Figure 2(a)) shows that the N-C-800 particle is formed by gathering the interconnected amorphous carbon layers, which is a highly curled layer analogous to graphene [22]. As shown in Figure 2(b), the lattice distance is 0.34 nm, which is consistent with the (002) crystalline plane of graphitized carbon. Moreover, many meso- and micropores (dashed lines in Figure 2(b)) can be observed in this material. Elemental mapping analysis of N-C-800 catalyst was also conducted to obtain the distribution of C, N and O elements. As shown in Figures 2(c)-(f), it can be seen that three elements are homogeneously distributed throughout the N-C-800 particle and the distribution dense of N element is high, suggesting that nitrogen element is uniformly doped into the carbon network.
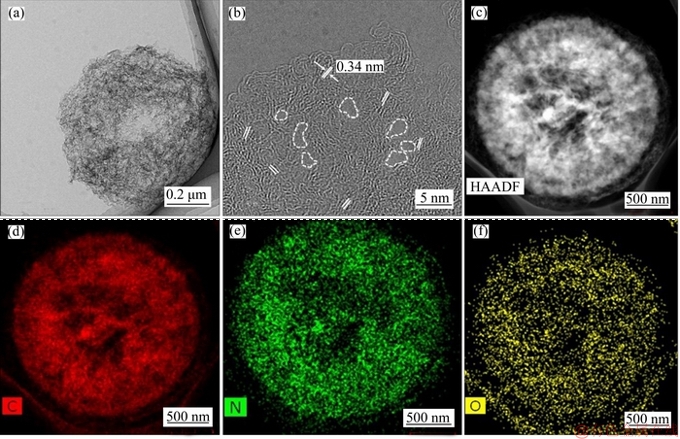
Figure 2 TEM (a) and HRTEM (b) images of N-C-800 catalyst, HAADF-STEM image of N-C-800 catalyst (c) and corresponding elemental mapping of C (d), N (e) and O (f)
The nitrogen adsorption-desorption isotherm curve of N-C-800 catalyst is shown in Figure 3. According to international union of pure and applied chemistry (IUPAC) classification, this sample shows type IV isotherms, suggesting meso- and micro-porous nature [27, 30-33], in good accordance with the SEM and TEM results. The BET specific surface area of the sample is calculated to be 631.46 m2/g. The high BET specific surface area and porosity are beneficial to the exposure of active sites and the adsorption of oxygen [10, 15].
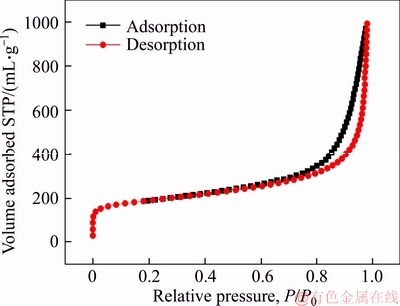
Figure 3 Nitrogen adsorption-desorption isotherms of N-C-800 catalyst
XPS measurements of N-C catalysts at different pyrolysis temperatures were carried out to further know the elemental composition, content and binding states of N in N-C materials. All the survey curves (Figure 4) of three samples exhibit the presence of C, N and O, which is consistent with the elemental mapping result. The overall N contents of N-C-600, N-C-700, N-C-800 are 25.41%, 12.44% and 9.81% (molar fraction), respectively, as revealed by the sharply decreased nitrogen characteristic peaks with increasing the pyrolysis temperature. The high-resolution N1s XPS spectra of all samples are divided into three types of N species: pyridinic N, pyrrolic N, graphitic N, which are usually thought to make a contribution to the electrocatalytic activity [33-38]. It is known that the pyridinic and pyrrolic N atoms are doped at vacancies or edges, while the graphitic N atoms directly substitute carbon atoms within graphene lattice [13, 39]. It is noteworthy that the proportion of graphitic N increases when enhancing the pyrolysis temperature, suggesting that the graphitic N possesses a better thermal stability compared to the other two types. The results above indicate that both the overall N content and the proportion of graphitic N can be tailored by controlling the pyrolysis temperature.
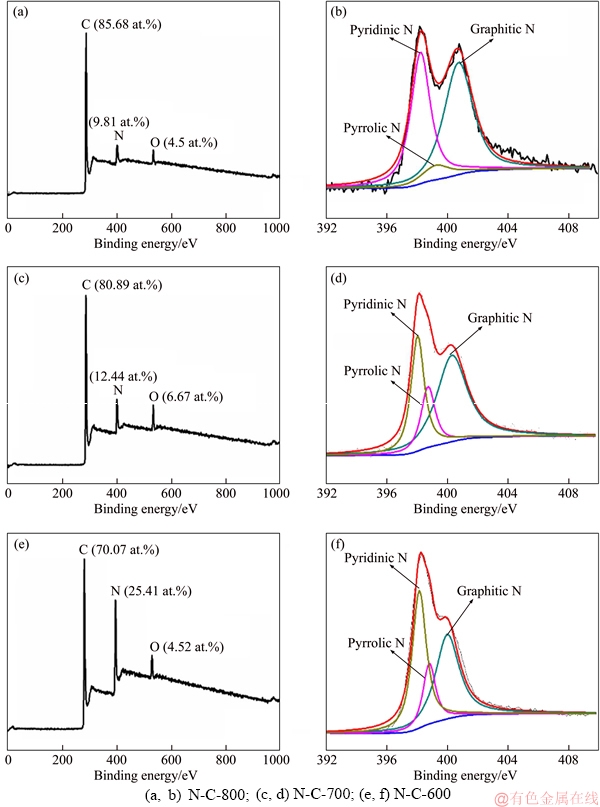
Figure 4 XPS survey (a, c, e) and high-resolution N 1s spectrum (b, d, f) of different materials:
Raman spectra of the as-synthesized samples at different temperatures were performed to understand the degree of disordered/ordered and defect in the material structure causing by N doping [4, 21, 33]. As shown in Figure 5(a), all Raman spectra of samples exhibit two evident peaks at about 1340 and 1588 cm-1, corresponding to D and G band, respectively. The intensity ratio of D and G band (ID/IG) can be used to evaluate the graphitization degree of materials [21, 26, 40]. The ID/IG value of samples synthesized at 600, 700, and 800 °C are calculated to be 1.22, 1.19, and 1.10, respectively. The result manifests that the N-C catalyst with a higher synthesized temperature shows a higher ordered structure and graphitization degree, which means a higher conductivity and structural stability during the ORR process. The doping of heteroatoms could introduce defects within graphene lattice. Based on the XPS results, the ascending ID/IG value with the decreasing temperatures possibly is ascribed to the increased defects caused by a higher concentration of N doping into the graphene lattice at a lower temperature.
Further structural information of catalysts at different pyrolysis temperatures was achieved from XRD patterns results (Figure 5(b)). All patterns display a typical broad peak at about 24°, which corresponds to the (002) crystal plane of graphitic carbon in relation to the extent of graphitization of materials [34, 41, 42]. Increasing the temperature from 600 to 700 °C, the (002) peak becomes sharper, indicating a higher degree of graphitization. This result is consistent with the above Raman results. Furthermore, there is another peak at about 43°, corresponding to the (101) peak of graphitic carbon. This peak indicates the order along the a-axis, which might offer a kind of steady conducting pathway inside the material [22].
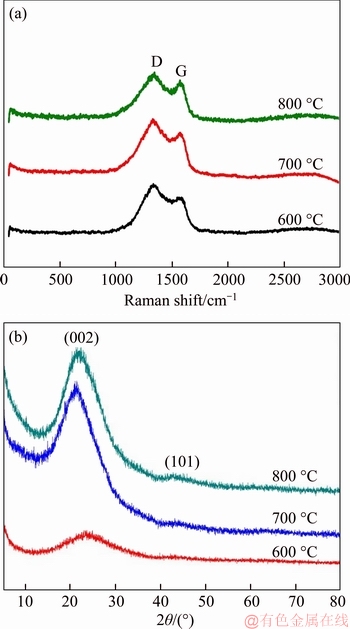
Figure 5 Raman spectra (a) and XRD patterns (b) of N-C materials prepared at different pyrolysis temperatures
The electrocatalytic activity of as-prepared catalysts for ORR was estimated based on the cyclic voltammetry (CV) measurements in O2-saturated 0.1 mol/L KOH electrolyte. CV curves of all samples are shown in Figure 6(a). It can be seen that the reductive peak (0.83 V) of N-C-800 catalyst can be comparable to the 20% Pt/C, which is far more positive than that of Glucose-800 without MCA template. Even in comparison with Fe-N-C-800 catalyst, the N-C-800 also exhibits more positive reduction peak, revealing the importance of blood cell-like morphology. To further investigate the ORR performance of the as-prepared catalysts, linear sweeping voltammogram (LSV) measurements performed on RRDE in oxygen-saturated 0.1 mol/L KOH electrolyte with a scan rate of 10 mV/s at 1600 r/min were received. As shown in Figure 6(b), both the onset potential (Eonset) and diffusion- limiting current density (iL) of Glucose-800 catalyst are very low, indicating a very poor electrocatalytic activity. When the MCA template is used, the as-prepared N-C-800 catalyst shows a much better performance, suggesting that the N-doping and construction of 3D porous morphology can significantly boost the ORR performance of carbon material. The Eonset (0.94 V) of N-C-800 can be comparable to that of 20 wt% Pt/C and the iL value (about 6 mA/cm2) is even higher, demonstrating a very impressive ORR activity for metal-free electrocatalysts [5, 15, 39]. It is well known carbon materials with the Fe and N co-doping could greatly improve its ORR activity. However, in the work, we find that the as-prepared Fe-N-C-800 delivers inferior ORR activity in comparison with N-C-800. It is worth noting that the morphology was severely damaged after the introduction of Fe, as seen in Figure 1(d). In turn, this result is a good evidence of supporting the importance of the red-blood-cell-like morphology. As a matter of fact, the red-blood- cell-like morphology, possesses abundant pores, which interconnects to be a 3D porous network structure. The fluffy and porous structure can be conductive to the exposure of active sites and transport of oxygen, thus greatly enhancing the electrocatalytic activity [35-44].

Figure 6 CV (a) and LSV (b) curves of as-prepared Glucose-800, N-C-800, Fe-N-C-800 catalysts and commercial 20% Pt/C
In order to gain more information about the electrocatalytic activity of as-prepared N-C catalysts, the CV and LSV curves of N-C catalysts at different pyrolysis temperatures are shown in Figure 7. For comparison, the electrocatalytic activity of N-C catalyst increases with the increasing temperature and reaches to the best when the pyrolysis temperature is 800 °C (N-C-800). However, the performance of the sample at 900 °C declines. At the meantime, both samples pyrolyzed at 600 °C (N-C-600) and 700 °C (N-C-700) show very poor electrocatalytic activity. It is noted that, from the results of XPS, the total N contents of the N-C-600 and N-C-700 catalysts are both very high and reach up to 25.41 at% and 12.44 at%, respectively. That is, the total N content of the N-C-600 is nearly three times than that of N-C-800 (9.81 at%). While, the electrocatalytic activity of N-C-800 catalyst is much better than that of N-C-600 and N-C-700, indicating that the electrocatalytic activity does not strongly depend on the total nitrogen content, but the nitrogen species.
To understand more about the ORR electrocatalytic mechanism of as-prepared N-C-800 catalyst, the rotating ring-disk electrode (RRDE) measurement was performed to accurately measure the yield of peroxide generated on the electrode during the ORR process. As shown in Figure 9, the tested H2O2 yield (%) of N-C-800 catalyst is less than 6% from 0.1 to 0.8 V, suggesting that a very low-level production of intermediate product was formed during ORR process catalyzed by as-prepared catalyst. The average electron transfer number (n) based on the calculation of RRDE test almost remains at 4.0 in the potential range from 0.1 to 0.8 V, indicating that the ORR process here is a 4e path with directly forming OH- as the final product. Compared to 2e process, the 4e process is normally considered as an ideal reaction path since the peroxide formed in 2e path would poison the catalysts [5, 10, 15, 45-48].
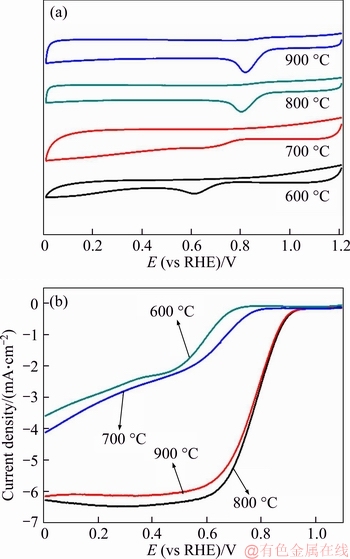
Figure 7 CV (a) and LSV curves (b) of N-C catalysts prepared at different temperatures
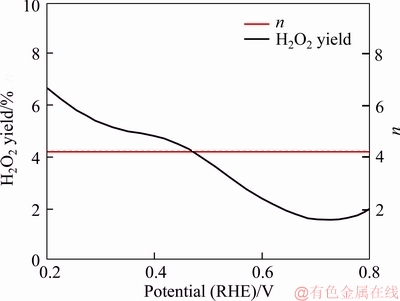
Figure 8 Hydrogen peroxide yield and electron transfer number (n) of N-C-800 electrode based on calculation of RRDE test
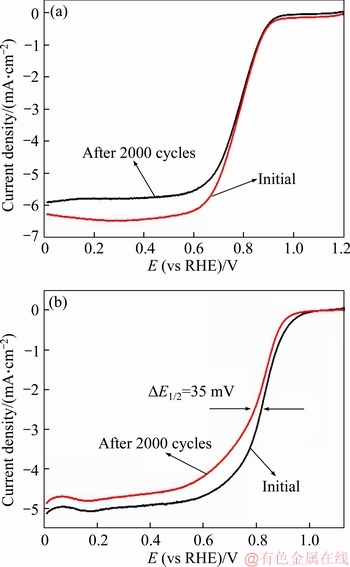
Figure 9 LSV curves of N-C-800 catalyst (a) and 20 wt% Pt/C (b) before and after 2000 cycles
Stability tests of N-C catalyst were conducted by cycling the as-prepared catalyst for 2000 cycles from 0.4 to 1.0 V with a sweeping speed of 200 mV/s in 0.1 mol KOH electrolyte saturated by oxygen. 20 wt% Pt/C was also performed as a reference. As shown in Figure 9, for N-C-800 catalyst, there is almost no shift of half-wave potential (E1/2) and the limiting current density is only reduced by 7.6%. For 20 wt% Pt/C, a negative shift of 35 mV and a reduction of limiting current density by around 5% under the same measurement conditions are clearly observed. The above results indicate that the N-C-800 catalyst shows a much better durability than 20 wt% Pt/C catalyst.
4 Conclusions
A kind of red-blood-cell-like N-C catalyst with 3D porous structure and high nitrogen content (9.81%) is synthesized by a facile self-sacrificial template method. The use of MCA template could generate a high nitrogen content and a novel 3D porous network. And the effective N doping could greatly improve the electrocatalytic activity of N-C catalyst. The as-prepared catalyst exhibited a comparable electrocatalytic activity for ORR and a higher durability in alkaline solution than 20 wt% Pt/C catalyst, and the ORR process catalyzed by as-prepared N-C catalyst is a 4e pathway. The high specific surface area and the abundant porous network structure are believed to be conducive to the exposure of active sites, the absorption and storage of oxygen, and the transport of electrons, thus resulting in superior ORR performance. It is also found that the electrocatalytic performance of the N-C catalysts depends on the proportion of graphitic N, rather than the total nitrogen content.
References
[1] GU Ying-ying, ZHAO Li, YANG Ming-yang, XIONG Yi-qiu, WU Zhe, ZHOU Min-jia, YAN Jun. Preparation and characterization of highly photocatalytic active hierarchical BiOX (X=Cl, Br, I) microflowers for rhodamine B degradation with kinetic modelling studies [J]. Journal of Central South University, 2017, 24(4): 754-765.
[2] PENG Hong-jian, XIAO Li-hong, CAO Yuan-ni, LUAN Xiang-feng. Synthesis and ionic conductivity of Li6La3BiSnO12 with cubic garnet-type structure via solid-state reaction [J]. Journal of Central South University, 2015, 22(8): 2883-2886.
[3] DAI Li-ming, XUE Yu-hua, QU Liang-ting, CHOI H J, BAEK J B. Metal-free catalysts for oxygen reduction reaction [J]. Chem Rev, 2015, 115(11): 4823-4892.
[4] ZHANG Jin-tao, LI Dai. Heteroatom-doped graphitic carbon catalysts for efficient electrocatalysis of oxygen reduction reaction [J]. ACS Catal, 2015, 5(12): 7244-7253.
[5] LI Jing-sha, CHEN Jia-jie, WAN Hao, XIAO Jin, TANG You-gen, LIU Min, WANG Hai-yan. Boosting oxygen reduction activity of Fe-N-C by partial copper substitution to iron in Al-air batteries [J]. Applied Catalysis B: Environmental, 2019, 242: 209-217.
[6] CHENG Fang-yi, CHEN Jun. Metal-air batteries: From oxygen reduction electrochemistry to cathode catalysts [J]. Chem Soc Rev, 2012, 41: 2172-2192.
[7] WEI Da-cheng, LIU Yun-qi, WANG Yu, ZHANG Hong-liang, HUANG Li-ping, YU Gui. Synthesis of N doped graphene by chemical vapor deposition and its electrical properties [J]. Nano Lett, 2009, 9(5): 1752-1758.
[8] SHENG Zhen-huan, SHAO Lin, CHEN Jing-jing, BAO Wen-jing, WANG Feng-bin, XIA Xing-hua. Catalyst-free synthesis of nitrogen-doped graphene via thermal annealing graphite oxide with melamine and its excellent electrocatalysis [J]. ACS Nano, 2011, 5(6): 4350-4358.
[9] DENG Hai-jing, LI Qian, LIU Jing-jun, WANG Feng. Active sites for oxygen reduction reaction on nitrogen-doped carbon nanotubes derived from polyaniline [J]. Carbon, 2017, 112: 219-229.
[10] LI Jing-sha, ZHOU Zhi, LIU Kun, LI Fu-zhi, PENG Zhi-guang, TANG You-gen, WANG Hai-yan. Co3O4/Co-N-C modified ketjenblack carbon as an advanced electrocatalyst for Al-air batteries [J]. Journal of Power Sources, 2019, 343: 30-38.
[11] WANG Zhong-li, XU Dan, XU Ji-jing, ZHANG Xin-bo. Oxygen electrocatalysts in metal-air batteries: From aqueous to nonaqueous electrolytes [J]. Chem Soc Rev, 2014, 43: 7746-7786.
[12] CAO Rui-guo, LEE J S, LIU Mei-lin, CHO J. Non-precious catalysts: Recent progress in non-precious catalysts for metal-air batteries [J]. Adv Energy Mater, 2012, 2: 816-829.
[13] DING Wei, LI Li, XIONG Kun, WANG Yao, LI Wei, NIE Yao, CHEN Si-guo, QI Xue-qiang, WEI Zi-dong. Shape fixing via salt recrystallization: A morphology-controlled approach to convert nanostructured polymer to carbon nanomaterial as a highly active catalyst for oxygen reduction reaction [J]. J Am Chem Soc, 2015, 137: 5414-5420.
[14] LIN Hua, CHEN Kang-hua, SHUAI Yi, HE Xuan, GE Ke. Influence of CsNO3 as electrolyte additive on electrochemical property of lithium anode in rechargeable battery [J]. Journal of Central South University, 2018, 25(4): 719-728.
[15] LI Jing-sha, CHEN Jia-jie, WANG Hai-yan, REN Yu, LIU Kun, TANG You-gen, LIU Min. Fe/N Co-doped carbon materials with controllable structure as highly efficient electrocatalysts for oxygen reduction reaction in Al-air batteries [J]. Energy Storage Materials, 2017, 8: 49-58.
[16] WANG Zhi-juan, CAO Xie-hong, PING Jian-feng, WANG Yi-xian, LIN Ting-ting, HUANG Xiao, MA Qing-lang, WANG Fu-ke, HE Chao-bin, ZHANG Hua. Electrochemical doping of three-dimensional graphene networks used as efficient electrocatalysts for oxygen reduction reaction [J]. Nanoscale, 2015, 7: 9394-9398.
[17] WANG Qi-bin, MA Hong-bo, KONG Xian-guang, ZHANG Yi-min. A distributed dynamic mesh model of a helical gear pair with tooth profile errors [J]. Journal of Central South University, 2018, 25(2): 287-303.
[18] ZHAI Yun-pu, DOU Yu-qian, ZHAO Dong-yuan, FULVIO P F, MAYES R T, DAI Shen. Carbon materials for chemical capacitive energy storage [J]. Adv Mater, 2011, 23: 4828-4850.
[19] YAN Jing, MENG Hui, XIE Fang-yan, YUAN Xiao-li, YU Wen-dan, LIN Wo-rong, OUYANG Wen-peng, YUAN Ding-sheng. Metal-free nitrogen-doped hollow mesoporous graphene-analogous spheres as effective electrocatalyst for oxygen reduction reaction [J]. J Power Source, 2014, 245: 772-778.
[20] NIU Wen-han, LI Li-gui, LIU Xiao-jun, WANG Nan, LIU Ji, ZHOU Wei-jia, TANG Zheng-hua, CHEN Shao-wei. Mesoporous N-doped carbons prepared with thermally removable nanoparticle templates: An efficient electrocatalyst for oxygen reduction reaction [J]. J Am Chem Soc, 2015, 137: 5555-5562.
[21] NIE Yao, LI Li, WEI Zi-dong. Recent advancements in Pt and Pt-free catalysts for oxygen reduction reaction [J]. Chem Soc Rev, 2015, 44: 2168-2201.
[22] WU Qiu-mei, RUAN Jian-ming, ZHOU Zhong-cheng, SANG Shang-bin. Magneli phase titanium sub-oxide conductive ceramic TinO2n-1 as support for electrocatalyst toward oxygen reduction reaction with high activity and stability [J]. Journal of Central South University, 2015, 22(4): 1212-1219.
[23] MALDONADO S, STEVENSON K J. Influence of nitrogen doping on oxygen reduction electrocatalysis at carbon nanofiber electrodes [J]. J Phys Chem B, 2005, 109: 4707-4716.
[24] WANG Xiao-xia, ZOU Biao, DU Xin-xin, WANG Jian-nong. N-doped carbon nanocages with high catalytic activity and durability for oxygen reduction [J]. J Mater Chem A, 2015, 3(23): 12427-12435.
[25] LIU Ming-kai, SONG Yan-fang, HE Si-xin, PAN Ji-sheng, XIA Yong-yao, LIU Tian-xin. Nitrogen-doped graphene nanoribbons as efficient metal-free electrocatalysts for oxygen reduction [J]. ACS Appl Mater Interfaces, 2014, 6(6): 4214-4222.
[26] YU Ding-shan, ZHANG Qiang, DAI Li-ming. Highly efficient metal-free growth of nitrogen-doped single-walled carbon nanotubes on plasma-etched substrates for oxygen reduction [J]. J Am Chem Soc, 2010, 132(43): 15127-15129.
[27] JIANG Wen-jie, HU Jin-song, ZHANG Xing, JIANG Yan, YU Bin-bin, WEI Zi-dong. In-situ nitrogen-doped nano-porous carbon nanocables as an efficient metal-free catalyst for oxygen reduction reaction [J]. J Mater Chem A, 2014, 2: 10154-10160.
[28] CHEN Ping, XIAO Tian-yuan, QIAN Yu-hong, LI Shan-shan, YU Shu-hong. A nitrogen-doped graphene- carbon nanotube nanocomposite with synergistically enhanced electrochemical activity [J]. Adv Mater, 2013, 25: 3192-3196.
[29] QU De-yang. Investigation of oxygen reduction on activated carbon electrodes in alkaline solution [J]. Carbon, 2007, 45: 1296-1301.
[30] WONG Wai-yin, DAUD W R W, MOHAMAD A B, KADHUM A A H, LOH K S, MAJLAN E H. Influence of nitrogen doping on carbon nanotubes towards the structure, composition and oxygen reduction reaction [J]. Int J Hydrogen Energy, 2013, 38: 9421-9430.
[31] YANG Zhi, NIE Hua-gui, CHEN Xi-an, CHEN Xiao-hua, HUANG Shao-ming. Recent progress in doped carbon nanomaterials as effective cathode catalysts for fuel cell oxygen reduction reaction [J]. J Power Sources, 2013, 236: 238-249.
[32] LIU Sheng-wen, ZHANG Hai-min, ZHAO Qian, ZHANG Xian, LIU Rong-rong, GE Xiao, WANG Guo-zhong, ZHAO Hui-jun, CAI Wei-ping. Metal-organic framework derived nitrogen-doped porous carbon@graphene sandwich-like structured composites as bifunctional electrocatalysts for oxygen reduction and evolution reactions [J]. Carbon, 2016, 106: 74-83.
[33] CHEN Ping, WANG Li-kun, WANG Gan, GAO Min-gui, GE Jin, YUAN Wen-jin. Nitrogen-doped nanoporous carbon nanosheets derived from plant biomass: An efficient catalyst for oxygen reduction reaction [J]. Energy Environ Sci, 2014, 7: 4095-4103.
[34] QIAN Ying-dan, DU Pan, WU Ping, CAI Chen-xin, GERVASIO D F. Chemical nature of catalytic active sites for the oxygen reduction reaction on nitrogen-doped carbon- supported non-noble metal catalysts [J]. J Phys Chem C, 2016, 120: 9884-9896.
[35] YANG Xiao-ling, ZOU Wen-jian, SU Yun-he, ZHU Yi-hua, JIANG Hong-liang, SHEN Jian-hua. Activated nitrogen- doped carbon nanofibers with hierarchical pore as efficient oxygen reduction reaction catalyst for microbial fuel cells [J]. J Power Sources, 2014, 266: 36-42.
[36] ZHANG Jun-li, CHEN Gao-li, ZHANG Qian, KANG Fei, YOU Bo. Self-assembly synthesis of N-doped carbon aerogels for supercapacitor and electrocatalytic oxygen reduction [J]. ACS Appl Mater Interfaces, 2015, 7: 12760-12786.
[37] LIANG Ji, DU Xin, GIBSON C, DU Xi-wen, QIAO Shi-zhang. N-doped graphene natively grown on hierarchical ordered porous carbon for enhanced oxygen reduction [J]. Adv Mater, 2013, 25: 6226-6231.
[38] SUN Yi-qing, LI Chun, SHI Gao-quan. Nanoporous nitrogen-doped carbon modified graphene as electrocatalyst for oxygen reduction reaction [J]. J Mater Chem, 2012, 22(25): 12810-12816.
[39] LAI Lin-fei, POTTS J R, ZHAN Da, WANG Liang, POH C K, TANG Chun-hua, GONG Hao, SHEN Ze-xiang, LIN Jian-yi, RUOFF R S. Exploration of the active center structure of nitrogen-doped graphene-based catalysts for oxygen reduction reaction [J]. Energy Environ Sci, 2012, 5: 7936-7942.
[40] LIU Rui-li, WU Dong-qing, FENG Xin-liang, MLLEN K. Nitrogen-doped ordered mesoporous graphitic arrays with high electrocatalytic activity for oxygen reduction [J]. Angew Chem Int Ed, 2010, 122: 2619-2623.
[41] WIGGINS-CAMACHO J D, STEVENSON K J. Mechanistic discussion of the oxygen reduction reaction at nitrogen-doped carbon nanotubes [J]. J Phys Chem C, 2011, 115(40): 20002-20010.
[42] YANG Wen, FELLINGER T P, Antonietti M. Efficient metal-free oxygen reduction in alkaline medium on high-surface-area mesoporous nitrogen-doped carbons made from ionic liquids and nucleobases [J]. J Am Chem Soc, 2011, 133: 206-209.
[43] LI Jing-sha, FU Liang, LUAN Jing-yi, XIE Hua-lin, CHENG Fang-yi, TANG You-gen, WANG Hai-yan. A strategy to achieve well-dispersed hollow nitrogen-doped carbon microspheres with trace iron for highly efficient oxygen reduction reaction in Al-air batteries [J]. J Electrochem Soc, 2018, 165: A3766-A3772.
[44] LIU De-pei, FU Liang, HUANG Xiao-bing, LIU Kun, LI Jing-sha, XIE Hua-lin, TIAN Jing, WANG Hai-yan, Tang You-gen. Influence of iron source type on the electrocatalytic activity towards oxygen reduction reaction in Fe-N/C for Al-air batteries [J]. J Electrochem Soc, 2018, 165(9): F662-F670.
[45] SONG Jing-ya, REN Yu-rong, LI Jing-sha, HUANG Xiao-bing, CHENG Fang-yi, TANG You-gen, Wang Hai-yan. Core-shell Co/CoNx@C nanoparticles enfolded by Co-N doped carbon nanosheets as a highly efficient electrocatalyst for oxygen reduction reaction [J]. Carbon, 2018, 138: 300-308.
[46] WANG Li-ping, FU Liang, LI Jing-sha, ZENG Xian-guang, XIE Hua-lin, HUANG Xiao-bing, WANG Hai-yan, TANG You-gen. On an easy way to prepare highly efficient Fe/N-co-doped carbon nanotube/nanoparticle composite for oxygen reduction reaction in Al-air batteries [J]. J Mater Sci, 2018, 53: 10280-10291.
[47] LI Jing-sha, ZHOU Nan, SONG Jing-ya, FU Liang, YAN Jun, TANG You-gen, WANG Hai-yan. Cu-MOF derived Cu/Cu2O nanoparticles and CuNxCy species to boost oxygen reduction activity of ketjenblack carbon in Al-air battery [J]. ACS Sus Chem Engin, 2018, 6: 413-421.
[48] LI Fu-zhi, LI Jing-sha, FENG Qiu-ju, YAN Jun, TANG You-gen, WANG Hai-yan. Significantly enhanced oxygen reduction activity of Cu/CuNxCy co-decorated ketjenblack catalyst for Al-air batteries [J]. J Energy Chem, 2018, 27: 419-425.
(Edited by FANG Jing-hua)
中文导读
血红细胞状氮掺杂多孔碳材料的制备及其氧还原催化性能的研究
摘要:以三聚氰胺氰尿酸为氮源和自牺牲模板,葡萄糖为碳源,采用两步法制备了一种高氮含量(9.81%)和高比表面积(631.46 m2/g)的血红细胞状氮掺杂多孔碳材料。该催化剂在碱性电解液中的起始电位和极限电流密度均优于商用20 wt% Pt/C 催化剂。该催化剂主要是通过四电子途径来催化氧还原反应过程。其优异的催化活性来源于原位掺杂的高含量氮与三维(3D)高比表面积多孔网络结构的协同作用,这有利于活性位点的暴露。有趣的是,氧还原的催化活性很大程度上取决于石墨氮的比例,而不是氮元素的总含量。
关键词:氧还原反应;氮掺杂碳;多孔结构;血红细胞状形貌
Foundation item: Projects(21571189, 21771062) supported by the National Natural Science Foundation of China; Projects(2016TP1007, 2017TP1001) supported by the Hunan Provincial Science and Technology Plan, China; Project(150110005) supported by the Fundamental Research and Innovation Project for Postgraduate of Hunan Province, China; Projects(2016CL04, 2017CL17) supported by the Opening Project of Material Corrosion and Protection Key Laboratory of Sichuan Province, China
Received date: 2019-02-25; Accepted date: 2019-03-27
Corresponding authors: TANG You-gen, PhD, Professor; Tel: +86-13607315350; E-mail: ygtang@csu.edu.cn; ORCID: 0000-0002- 7150-7947; PENG Zhi-guang, PhD, Associate Professor; Tel: +86-13170406770; E-mail: zhgpeng@csu.edu.cn; ORCID: 0000-0001-9559-7222

