DOI: 10.11817/j.issn.1672-7207.2017.09.002
选择性除去氯化钴溶液中铜的热力学分析
李孟春1,钱振1,车键勇1,陈爱良1,马玉天2,张燕2
(1. 中南大学 冶金与环境学院,湖南 长沙,410083;
2. 金川集团有限公司 贵金属厂,甘肃 金昌,737100)
摘要:根据溶液体系电荷平衡和离子同时平衡原理,通过热力学平衡计算,研究298 K下氯化钴溶液中Co-Cu(Ⅱ)-Cl-H2O体系金属离子及其配合离子浓度随pH及总氯浓度变化规律,并基于不同的除铜方法进行热力学分析。在Co-Cu(Ⅱ)-Cl-S-H2O体系中,计算硫化除铜终点各离子的平衡浓度,同时给出除铜终点金属浓度随总硫浓度对数变化关系图和随pH变化关系图。研究结果表明:在Co-Cu(Ⅱ)-Cl-H2O体系下,铜钴主要以阳离子配合物形式存在,加入还原剂后,在Co-Cu(Ⅰ)-Cl-H2O体系中,铜主要以阴离子配合物形式存在,钴仍主要以金属自由阳离子形式存在。因此,可以采用阴离子交换树脂深度除铜。随着总硫浓度的增加,铜逐渐沉淀,在除铜终点处恰好能保证钴不沉淀,因此,也可以采用硫化法除铜。
关键词:氯化钴溶液;选择性;热力学分析;硫化法;除铜
中图分类号:TF816 文献标志码:A 文章编号:1672-7207(2017)09-2264-07
Thermodynamic analysis on removal of copper selectively from cobalt chloride solution
LI Mengchun1, QIAN Zhen1, CHE Jianyong1, CHEN Ailiang1, MA Yutian2, ZHANG Yan2
(1. School of Metallurgy and Environment, Central South University, Changsha 410083, China;
2. Noble Metal Factory, Jinchuan Group Co.Ltd., Jinchang 737100, China)
Abstract: The diagrams of the concentration of Cu2+/Co2+ ions and/or their coordination compounds combined with Cl- were respectively drawn according to the thermodynamic equilibrium principle of charge, material and ions at 298 K. It reveals the change discipline of complex ions concentration with change of pH and cT(Cl). Based on the different Cu-removal methods, the thermodynamic was analyzed. In the system of Co-Cu(Ⅱ)-Cl-H2O, the balance concentration of ions at the point of final Cu-removal was calculated and the concentration logarithm diagrams of total metals and sulfur & pH were also obtained. The results show that the copper and cobalt mostly exist in the form of cation ions in the system of Co-Cu(Ⅱ)-Cl-H2O. But copper and cobalt exist as complex anion and free cation, respectively, after adding reducing reagent. Therefore, copper can be removed with anion exchange resin. Copper can be precipitated gradually with the increase of total sulfur concentration, which guarantees no precipitation of cobalt occurs at the point of final Cu-removal. Cu-removal from cobalt solution can be obtained by sulfide way.
Key words: cobalt chloride solution; selective; thermodynamics analysis; sulfide way; Cu-removal
金属钴因具有延展性和铁磁性等特性而被应用于工具钢、高性能耐热合金以及硬质合金等,近年来含钴化合物多用于电池材料,当前热门的锂电新能源已成为钴最大的消费领域[1]。在矿产资源中,由于铜钴性质相近,通常伴生存在,在钴冶炼过程中会跟随钴一起进入氯化钴溶液中。如果氯化钴溶液中有一定量的铜存在时,因铜的电极电势比钴的正,那么在电解时铜会优于钴在阴极析出,影响电钴的质量。为了不影响电钴质量,行业要求钴电解液中铜的浓度小于4.72×10-5 mol/L[2](质量浓度约为3 mg/L)。常用的除铜的方法有离子交换法、萃取法、电解法、硫化法等。其中,选用弱碱性阴离子交换树脂深度除杂净化电解液,得到了质量分数为99.9995%高纯钴[3-4]。该技术工艺简单,可实现无渣除杂,但是,离子交换法在后续的解吸过程中耗水量大,在解吸再生循环过程中,可能会降低树脂的交换容量,从而会增加后续的处理负担。采用饱和负载盐酸的N235萃取还原后钴电解阳极液中的铜[5-6],并达到了预期效果,但是该工艺需要多级萃取才能深度去除,且在溶液中会夹杂有机相。国内许多研究者提出电沉积法除铜,生产出含铜(质量分数)95%、镍低于1%的铜粉,大大提高了镍的直收率[7-9]。但是采用此法用于钴电解液中,不能达到深度除铜的目的,且该过程电能消耗高,溶液处理量小,不适合大规模生产。与其他方法相比,硫化法具有明显的优势。将10%的硫化钠溶液加入反应釜中,加热至40~50 ℃并搅拌,可使铜铅以硫化物的形态除去[10-13]。该法因操作方便、试剂便宜易得而受人青睐。目前,已有多种方法面向从氯化钴溶液中除铜的应用研究,但对该溶液的理论研究较少。鉴于氯化钴溶液中金属离子与配位体之间会形成多级配合物,因此,本文作者根据电荷平衡、离子同时平衡原理,进行氯化钴溶液Co-Cu(Ⅱ)-Cl-S-H2O系及Co-Cu(Ⅰ)-Cl-S- H2O系热力学研究,并结合文献和实验结果对其热力学分析结果进行验证。
1 热力学分析
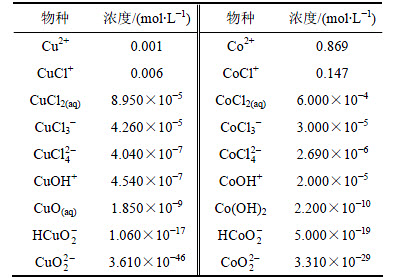
本文研究热力学的对象是含铜的氯化钴溶液,其主要成分如表1所示。从表1可以看出,溶液属钴高铜低的氯化盐体系。
1.1 Co-Cu-Cl-H2O体系热力学分析
在氯离子浓度为2.05 mol/L的氯化体系中,氯离子易与钴、铜发生反应形成氯离子配合物。根据离子同时平衡原理,其配位反应为
表1 氯化钴溶液成分
Table 1 Chemical composition of cobalt chloride solution mmol/L

Me2++iL-=MeLi2-i (1)
式中:i为配位数,i=1,2,3,4;L为Cl;Me为Cu和Co。
其配位稳定常数βi为
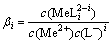 (2)
(2)
除了上述反应外,Co2+和Cu2+会和OH-发生反应生成配合物,金属离子与OH-的配位稳定常数 为:
为:
nH2O+Me2+=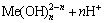
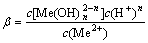 (3)
(3)
2H2O+Me2+= +3H+
+3H+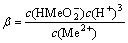 (4)
(4)
H2O+Me2+=MeO(aq)+2H+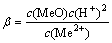 (5)
(5)
2H2O+Me2+=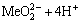
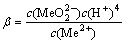 (6)
(6)
每种金属与氯离子和氢氧根离子反应生成各级配合物的累积稳定常数如表2所示。
表2 各离子配位累积稳定常数[14-16]
Table 2 Accumulative equilibrium constant of coordination complexes[14-16]

根据电荷平衡和离子同时平衡原理[17-19],钴、铜、氯的总量在整个反应过程中恒定不变,反应进行到一定阶段就会达到近似平衡状态,设cT(Co),cT(Cu)和cT(Cl)分别为钴、铜、氯的总浓度,自由离子及其配位物的浓度总和为:
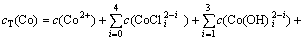
 (7)
(7)
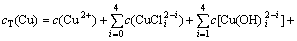
 (8)
(8)
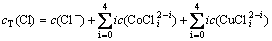 (9)
(9)
根据电荷平衡,有
c(Cl-)+c(OH-)+c(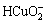 )+c(
)+c( )+c(
)+c( )+
)+
c(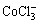 )+[2×[c(
)+[2×[c(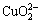 )+c(
)+c(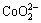 )+c(
)+c(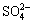 )+
)+
c(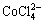 )+c(
)+c(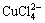 )]=c(H+)+c(Na+)+c(CoCl+)+
)]=c(H+)+c(Na+)+c(CoCl+)+
c(CuCl+)+c(CoOH+)+c(CuOH+)+2×[c(Cu2+)+c(Co2+)] (10)
根据文献[13]报道,在pH=4.5时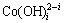 浓度极低(多种配合物浓度在10-30 mol/L以下), 即便提高OH-的浓度,
浓度极低(多种配合物浓度在10-30 mol/L以下), 即便提高OH-的浓度,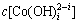 也仍然保持在一个较低的水平,所以,在较低的pH(pH<7)的情况不予考虑。
也仍然保持在一个较低的水平,所以,在较低的pH(pH<7)的情况不予考虑。
在c(Cl)T=2.05 mol/L,cT(Cu)=7.87 mmol/L和cT(Co)=1.017 mol/L时,采用文献报道的热力学分析方法[17],计算出pH在1~8时铜钴和氯配合物浓度与pH的关系,见图1。从图1可以看出:在这些配合物中,由于Cl-的配合作用,当pH<5.8时会使得溶液中的铜大部分以CuCl+的形式存在;随着pH升高,氯化钴溶液中的OH-浓度开始增加;当pH>5.8时,在计算过程中Qsp(Qsp=c(Cu2+)c(OH-)2)等于Cu(OH)2的Ksp(溶度积常数),说明有Cu(OH)2沉淀生成,此时,铜和氯形成的各种配合物的浓度迅速降低。从图2可以看出:在氯化体系中,当pH<6.5时钴主要以Co2+存在,CoCl+次之;当pH>6.5时,Qsp(Qsp=c(Co2+)c(OH-)2)等于Co(OH)2的Ksp,生成Co(OH)2沉淀,此时钴的各种配合物浓度也同样迅速降低。
从以上热力学分析可以得出:在氯化体系中,铜钴都会与氯以阳离子形式大量存在,进而无法用常规的离子交换树脂进行深度除铜。Co-Cu(Ⅰ)-Cl-H2O系中各铜氯配合离子浓度与pH的关系及其分配情况如图3所示。从图3可以看出:Cu+的氯化体系中,铜主要以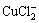 和
和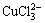 的形式存在,其
的形式存在,其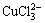 的最高,且铜基本不会以Cu+形式存在。可见,若加入还原剂,将Cu2+还原成Cu+,铜主要以铜氯络合阴离子形式存在,而钴依然以Co2+形式存在,故可以采用阴离子交换树脂深度除去氯化钴溶液中的铜。与镍电解液相似,文献[12]报道在氯化体系中先将Cu2+还原成Cu+,再用阴离子交换树脂除去镍电解液中的铜,达到了电解液要求的除铜深度,同时也验证了上述分析结果。
的最高,且铜基本不会以Cu+形式存在。可见,若加入还原剂,将Cu2+还原成Cu+,铜主要以铜氯络合阴离子形式存在,而钴依然以Co2+形式存在,故可以采用阴离子交换树脂深度除去氯化钴溶液中的铜。与镍电解液相似,文献[12]报道在氯化体系中先将Cu2+还原成Cu+,再用阴离子交换树脂除去镍电解液中的铜,达到了电解液要求的除铜深度,同时也验证了上述分析结果。
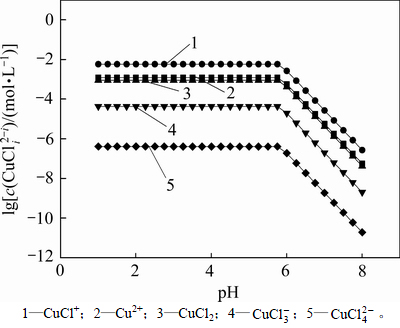
图1 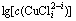 -pH的关系
-pH的关系
Fig. 1 Relationship between 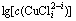 and pH
and pH
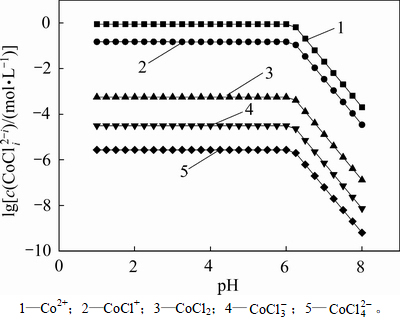
图2 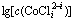 -pH的关系
-pH的关系
Fig. 2 Relationship between 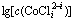 and pH
and pH
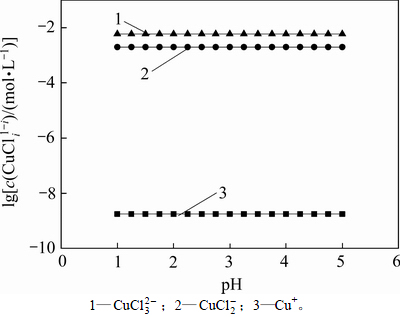
图3 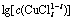 -pH的关系
-pH的关系
Fig. 3 Relationship between 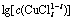 and pH
and pH
在pH=4.5,cT(Cu)=7.87 mmol/L和cT(Co)=1.017 mol/L时,计算出不同总氯浓度下Co-Cu-Cl-H2O系的氯化钴溶液中金属及配合离子的浓度分布,如图4和5所示。从图4可以看出:随着溶液中总氯浓度的增加,Co2+与Cl-形成配合离子机会多,形成的钴氯络合物增多,导致Co2+的浓度呈缓慢下降趋势,其他络合物的浓度呈上升趋势。
从图5可以看出:随着总氯浓度的增加,Cu2+的浓度逐渐降低,CuCl2和CuCl+浓度逐渐升高,CuCl+浓度从2.84 mmol/L升高至5.74 mmol/L,当总氯浓度从2 mol/L增加到5 mol/L时,其浓度又从5.74 mmol/L降低至5.21 mmol/L,若继续增大氯离子浓度,CuCl+浓度将继续减少,且其他配合物浓度将逐渐增加。原因可能是随着总氯浓度增加,Cl-与Cu2+多级配位的能力提高,使得 和
和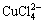 浓度开始有明显上升,从而导致CuCl+浓度有所下降。
浓度开始有明显上升,从而导致CuCl+浓度有所下降。
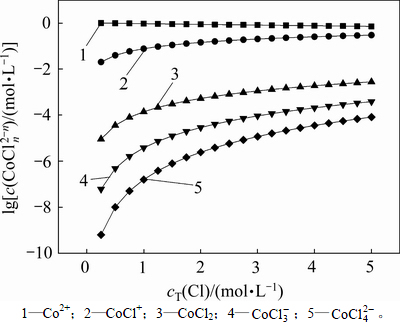
图4 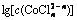 -
- 的关系
的关系
Fig. 4 Relationship between 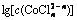 and
and 

图5 不同含铜物种与氯离子总浓度的关系
Fig. 5 Relationship between 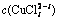 and
and 
某厂现场溶液体系(pH=4.5,cT(Cl)=2.05 mol/L,c(Cu)=7.87 mmol/L,c(Co)=1.017 mol/L,pH=4.5),通过计算得到氯化钴溶液中各金属离子及配合物的平衡浓度,如表3所示。从表3可以得到:铜在氯化体系中主要以CuCl+存在,其次为Cu2+,其他配合物含量很低。钴在氯化体系中大部分以Co2+为主,其次以CoCl+为主。
表3 pH=4.5时氯化钴溶液中各离子平衡浓度
Table 3 Equilibrium concentration of ions at pH=4.5
1.2 Co-Cu-Cl-S-H2O体系热力学分析
运用硫化法除氯化钴溶液中铜时,由于硫元素的引入体系中会有硫离子、硫氢根离子以及硫化氢,如式(11)和式(12)[18]所示:
H2S=H++HS-, K1=10-8.02 (11)
HS-=H++S2-, K2=10-12.88 (12)
其中生成的HS-会与Co2+和Cu2+按照式(13)~(16)生成相应的配位离子。
Cu2++ HS-=CuHS+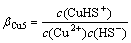 (13)
(13)
Cu2++2HS-=Cu(HS)2(aq)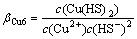 (14)
(14)
Co2++HS-=CoHS+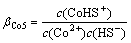 (15)
(15)
Co2++2HS-=Co(HS)2(aq)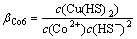 (16)
(16)
根据质量守恒定律,加入硫化物除铜剂除铜后,体系中总硫为
cT(S)=c(H2S)+c(S2-)+c(HS-)+c(CuHS+)+2c(Cu(HS)2(aq))+c(CoHS+)+2c(Co(HS)2(aq)) (17)
计算时,根据同时平衡原理,通过改变总硫浓度,经过反复迭代计算,得出当pH=4.5时,氯化钴溶液中金属浓度随总硫浓度的变化,如图6所示。
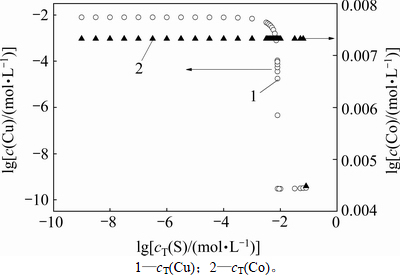
图6 金属浓度随总硫浓度的关系
Fig. 6 Relationship between metal concentration and sulfur concentration
从图6可以看出:当总硫量增加至10-9.5 mol/L时,c(Cu2+)和c(S2-)的离子积达到CuS溶度积,溶液中开始析出CuS;随着总硫浓度继续增加,当lg[cT(S)]≥ -2.106,即总硫浓度大于或等于7.83 mmol/L时,溶液中总铜浓度小于或等于3.57×10-5 mol/L,已达到氯化钴溶液中除铜标准(≤4.72×10-5 mol/L),其后继续增加总硫量。在保证铜离子沉淀而钴离子不沉淀的条件下,得到最佳除铜终点(硫离子与钴离子的乘积等于硫化钴的溶度积时即为终点)。
当cT(S)=6.31×10-2 mol/L,恰好能保证钴不沉淀,在此除铜终点处,列出主要离子的浓度,如表4所示。从表4可以看出:在保证Co不沉淀前提下,控制一定量的总硫,可以将Cu的浓度降至3.24×10-10 mol/L。其中铜主要以CuHS+和Cu(HS)2形式存在。
在除铜终点处,绘制出金属浓度随pH的变化关系如图7 所示。从图7可以看出:当pH=0~5.5时,随着pH的升高,氢离子浓度逐渐降低,使反应式(11)和式(12)向右进行,释放出更多的硫离子,使得溶液中铜浓度降低,提高了除铜深度。但在提高除铜深度的同时,也要保证钴不沉淀。
表4 除铜终点处各主要离子浓度
Table 4 Concentration of ions in equilibrium solution after deposition
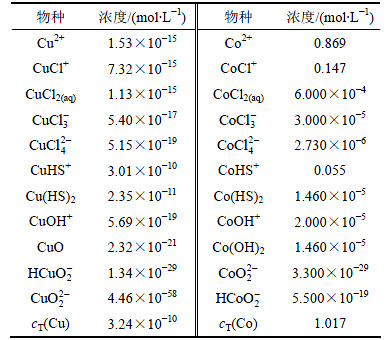
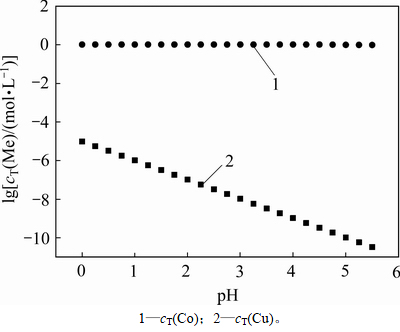
图7 除铜终点时金属浓度随pH的变化关系
Fig. 7 Relationship between equilibrium concentration of metal anions and pH
2 硫化法除铜实验
为了验证以上热力学分析结果,特进行氯化钴溶液的硫化法除铜验证实验。取氯化钴溶液100 mL到锥形瓶中,在pH=4.0,80 ℃,30 min条件下进行硫化钠用量的单因素实验,取样用ICP分析除铜后液中铜的浓度。
除铜后液铜含量变化结果如图8所示,其中过量系数为硫化钠与总铜的物质的量比。随着过量系数的增加,除铜后液中铜浓度逐渐减少,除铜后液中铜的浓度约为2.46×10-5 mol/L,达到电解液对铜杂质控制的要求(≤4.72×10-5 mol/L),而热力学计算得除铜后液含铜3.57×10-5 mol/L,都在控制范围内,理论计算结果较好地反映了实际情况。

图8 过量系数对除铜效率的影响
Fig. 8 Effect of different excess coefficient on Cu-removal
3 结论
1) Co-Cu(Ⅱ)-Cl-H2O系的氯化钴溶液中,铜主要以铜(Ⅱ)氯阳离子形式存在,而钴主要以阳离子形式存在,不能用常规的交换树脂选择性深度除铜。加入还原剂后,铜主要以铜(Ⅰ)氯阴离子配合物形式存在,而钴仍以阳离子形式存在,故可用阴离子交换树脂深度除铜。
2) 在用硫化法除铜的氯化钴Co-Cu(Ⅱ)-Cl-S- H2O体系中,当cT(S)=10-9.5 mol/L时,开始析出CuS沉淀;当继续增大总硫浓度至7.83 mmol/L时,溶液中铜浓度已降至3.57×10-5 mol/L,可以达到电解液的质量要求。在保证钴恰好不沉淀的同时,可以将铜继续降至3.24×10-10 mol/L。通过实验验证,证明了采用硫化法进行除铜具有可行性。
参考文献:
[1] 严康, 郭学益, 田庆华, 等. 中国锂离子电池系统钴代谢分析[J]. 中南大学学报(自然科学版), 2017, 48(1): 25-30.
YAN Kang, GUO Xueyi, TIAN Qinghua, et al. Cobalt flow analysis of lithium-ion battery system in China[J]. Journal of Central South University (Science and Technology), 2017, 48(1): 25-30.
[2] 何焕华, 蔡乔方. 中国镍钴冶金[M]. 北京: 冶金工业出版社, 2000: 465-469.
HE Huanhua, CAI Qiaofang. Nickel and cobalt metallurgy in China[M]. Beijing: Metallurgical Industry Press, 2000: 465-469.
[3] 温俊杰, 张启修, 张贵清, 等. 硅胶-聚合胺树脂在模拟硫酸镍电解液中深度净化除铜[J]. 有色金属, 2009, 61(1): 50-55.
WEN Junjie, ZHANG Qixiu, ZHANG Guiqing, et al. Deeply moving copper from cobalt chloride electrolyte with novel silica-polyamine resin[J]. Nonferrous metal, 2009, 61(1): 50-55.
[4] 刘丹, 贺昕, 熊晓东. 离子交换法深度除杂制备高纯钴的研究[J]. 稀有金属, 2013, 37(1): 112-115.
LIU Dan, HE Xin, XIONG Xiaodong. Preparation of high purity cobalt with deep purification by ion exchange[J]. Chinese Journal of Rare Metals, 2013, 37(1): 112-115.
[5] 楚广, 陈小红, 杨天足. 用Lix973选择性萃取氨性浸出液中的铜和钴[J]. 中南大学学报(自然科学版), 2016, 47(6): 1830-1834.
CHU Guang, CHEN Xiaohong, YANG Tianzu. Selective solvent extraction of copper and cobalt from ammonia leaching solution containing copper and cobalt using Lix973[J]. Journal of Central South University (Science and Technology), 2016, 47(6): 1830-1834.
[6] 哈敏. 溶剂萃取法净化钴电解阳极液研究[D]. 兰州: 兰州理工大学材料科学与工程学院, 2008: 13-15.
HA Ming. Solvent extraction as purification measurement of cobalt electrolyte[D]. Lanzhou University of Technology. School of Materials Science and Engineering, 2008: 13-15.
[7] 常全忠, 毛西康, 马岩, 等. 一种钴电解液的除铜方法: 中国, CN200410056895.7[P]. 2005-03-23.
CHANG Quanzhong, MAO Xikang, MA Yan, et al. A way of Cu-removal in the cobalt electrolyte: China, CN200410056895.7[P]. 2005-03-23.
[8] 王志兴, 李艳, 李新海, 等. 在氯离子氨性体系中电解分离铜、钴镍的方法及其产品的应用: 中国, CN201410317272.4[P]. 2014-11-05.
WANG Zhixin, LI Yan, LI Xinhai, et al. The way on the electrolysis separation of copper, cobalt, nickel in the chloride ion ammonia system and its application for products: China, CN 201410317272.4[P]. 2014-11-05.
[9] 曹振欧, 曾颖如, 吴鸿儒. 金川镍电解阳极液净化除铜的电沉积法研究[J]. 湖南冶金, 1997(4): 11-14.
CAO Zhenou, ZENG Yinru, WU Hongru. The study on Cu-removal from nickel anodic electrolyte using electro- deposited way[J]. Hunan Metallurgy, 1997(4): 11-14.
[10] 苏瑞娟. 镍电解液净化除铜工艺研究[D]. 甘肃: 兰州理工大学材料科学与工程学院, 2012: 15-16.
SU Ruijuan. Study on technology copper from nickel electrolyte solution[D]. Gansu: Lanzhou University of Technology. School of Materials Science and Engineering, 2012: 15-16.
[11] 赵中伟, 陈爱良, 孙培梅, 等. 镍电解阳极液深度除铜[J]. 中国有色金属学报, 2009, 19(4): 749-753.
ZHAO Zhongwei, CHEN Ailiang, SUN Peimei, et al. Removing copper from nickel electrolyte solution[J]. The Chinese Journal of Nonferrous Metals, 2009, 19(4): 749-753.
[12] 彭容秋. 镍冶金学[M]. 长沙: 中南大学出版社, 2005: 153-159.
PENG Rongqiu. Nickel metallurgy[M]. Changsha: Central South University Press, 2005: 153-159.
[13] 郭万根, 石玉臣, 王含渊, 等. Co-Cu-Zn-Pb-Ni-Cl-H2O体系的热力学分析[J]. 有色金属(冶炼部分), 2011(9): 5-8.
GUO Wangeng, SHI Yuchen, WANG Hanyuan, et al. Thermodynamic analysis of Co-Cu-Zn-Pb-Ni-Cl-H2O system[J]. Nonferrous Metal, 2011(9): 5-8.
[14] DEAN J A. 兰氏化学手册[M]. 尚久方, 译. 北京: 科学出版社, 1991: 231-246.
DEAN J A. Lange’s handbook of chemistry[M]. Shang Jiufang, trans. Beijing: Science Press, 1991: 231-246.
[15] 蒋明谦. 高等药物化学[M]. 北京: 科学出版社, 1958: 23-29.
JIANG Mingqian. Advanced medicinal chemistry[M]. Beijing: Science Press, 1958: 23-29.
[16] KAROLINA W, ALEKSANDRA W, MARTA K. Equilibrium and mechanism of cobalt(Ⅱ) extraction from chloride solution by hydrophobic 2-pyridineketoxime[J]. Separation and Purification Technology, 2015, 142: 129-136.
[17] 赵中伟, 胡宇杰, 李洪桂. 一种用EXECLE进行冶金热力学平衡计算的新方法[J]. 稀有金属与硬质合金, 2005, 33(1): 48-51.
ZHAO Zhongwei, HU Yujie, LI Honggui. A new method for metallurgical thermodynamic equilibrium calculation by EXCEL[J]. Rare Metals and Cemented Carbides, 2005, 33(1): 48-51.
[18] 刘承科. 大学化学[M]. 长沙: 中南工业大学出版社, 1994: 49-56.
LIU Chenke. College chemistry[M]. Changsha: Central South University of Technology Press, 1994: 49-56.
[19] 李绍英, 赵留成, 孙春宝, 等. 基于同时平衡原理的Au-I-H2O系热力学分析[J]. 中国有色金属学报, 2005, 25(7): 1987-1992.
LI Shaoyin, ZHAO Liucheng, SUN Chunbao, et al. Thermodynamic analysis for Au-I-H2O system based on principle of simultaneous equilibrium[J]. The Chinese Journal of Nonferrous Metals, 2005, 25(7): 1987-1992.
(编辑 赵俊)
收稿日期:2016-09-16;修回日期:2016-12-04
基金项目(Foundation item):国家自然科学基金资助项目(51104183);甘肃省科技重大专项计划项目(1602FKDC007) (Project(51104183) supported by the National Science Foundation of China; Project(1602FKDC007) supported by Science and Technology Major Program of Gansu Province)
通信作者:陈爱良,博士,副教授,从事有色金属冶金研究;E-mail: chenailiang@csu.edu.cn