苯乙烯膦酸体系中细粒金红石的疏水絮团浮选
来源期刊:中国有色金属学报(英文版)2018年第7期
论文作者:黄小涛 肖巍 赵红波 曹攀 胡奇秀 覃文庆 张雁生 邱冠周 王军
文章页码:1424 - 1432
关键词:疏水絮团浮选;细粒金红石;苯乙烯膦酸;DLVO理论
Key words:hydrophobic flocculation flotation; rutile fines; styryl phosphonic acid (SPA); DLVO theory
摘 要:通过浮选试验、动电位测试、显微镜观测、激光粒度分析、吸附量测定和DLVO理论研究细粒金红石在苯乙烯膦酸(SPA)体系中的絮团浮选行为。单矿物浮选试验结果表明,苯乙烯膦酸对细粒金红石的絮团浮选具有良好的诱导作用;同时,溶液pH值、剪切力(搅拌速率)和搅拌时间均对絮团浮选效果有一定影响。动电位测试发现,随着SPA的加入,等电点及电位均负向移动,表明矿物与药剂之间主要发生化学吸附。激光粒度分析表明,在搅拌速度为1800 r/min和1000 mg/L SPA时,金红石颗粒的尺寸最大。此外,通过显微镜观测和浮选试验证明絮团的产生有利于细粒金红石的浮选。综上可得,SPA通过化学吸附作用能有效诱导细粒金红石的疏水絮团并增大其颗粒尺寸。最后,通过DLVO理论计算进一步验证SPA 与金红石颗粒之间主要发生化学吸附作用,进而促进絮团产物的形成。
Abstract: The hydrophobic flocculation flotation of rutile fines in the presence of styryl phosphonic acid (SPA) was investigated by flotation tests, zeta-potential measurement, optical microscope observation, laser-based particle size analysis, adsorption measurements and DLVO theory. The flotation tests indicated that rutile fines could be flocculated by SPA, and pH, shear force (stirring speed) and stirring time played significant roles in flocculation. The isoelectric point (IEP) and zeta-potential in whole range all moved to negative values as SPA was added according to the results from zeta-potential measurement. It was demonstrated that the primary reason for above was chemical adsorption. The laser-based particle size results showed the particle size at a stirring speed of 1800 r/min and 1000 mg/L SPA was the largest in all experiments. Furthermore, using the optical microscope observation and flotation tests, it was important for flotation of rutile fines to produce the flocculant. In the light of above-mentioned facts, floc flotation of rutile fines could be induced in the form of chemical adsorption by SPA to increase particle size. The data calculated from DLVO theory also indicated that chemical adsorption was the main reason for the formation of flocculant.
Trans. Nonferrous Met. Soc. China 28(2018) 1424-1432
Xiao-tao HUANG1,2, Wei XIAO1,2, Hong-bo ZHAO1,2, Pan CAO1,2, Qi-xiu HU1,2, Wen-qing QIN1,2, Yan-sheng ZHANG1,2, Guan-zhou QIU1,2, Jun WANG1,2
1. School of Minerals Processing & Bioengineering, Central South University, Changsha 410083, China;
2. Key Laboratory of Biohydrometallurgy of Ministry of Education, Central South University, Changsha 410083, China
Received 17 January 2017; accepted 30 September 2017
Abstract: The hydrophobic flocculation flotation of rutile fines in the presence of styryl phosphonic acid (SPA) was investigated by flotation tests, zeta-potential measurement, optical microscope observation, laser-based particle size analysis, adsorption measurements and DLVO theory. The flotation tests indicated that rutile fines could be flocculated by SPA, and pH, shear force (stirring speed) and stirring time played significant roles in flocculation. The isoelectric point (IEP) and zeta-potential in whole range all moved to negative values as SPA was added according to the results from zeta-potential measurement. It was demonstrated that the primary reason for above was chemical adsorption. The laser-based particle size results showed the particle size at a stirring speed of 1800 r/min and 1000 mg/L SPA was the largest in all experiments. Furthermore, using the optical microscope observation and flotation tests, it was important for flotation of rutile fines to produce the flocculant. In the light of above-mentioned facts, floc flotation of rutile fines could be induced in the form of chemical adsorption by SPA to increase particle size. The data calculated from DLVO theory also indicated that chemical adsorption was the main reason for the formation of flocculant.
Key words: hydrophobic flocculation flotation; rutile fines; styryl phosphonic acid (SPA); DLVO theory
1 Introduction
Possessing the properties of high strength, low density and excellent corrosion resistance, titanium and its alloys have extensively been used as components in chemical processing equipment, and applied in many industries like aircraft, aero-engines and biomedical devices [1-4]. Rutile and ilmenite have served as important mineral materials for the production of titanium and its alloys. TiO2 as a low cost and high activity material has attracted great attention and application in photocatalytic research all over the world [5]. Thus, it is of remarkably practical significance to explore the development and utilization of natural rutile sand.
It is in a narrow particle size window that froth flotation is well responded to mineral processing. With the complicating of ore composition and the decrease in ore grade and particle size in the natural world, however, the effectiveness of flotation would dramatically decrease. Furthermore, most of the slimes will come into being during the process of mineral liberation [6]. It is generally accepted that the ore with the slimes will reduce concentrate grade and recovery [7]. Thus, it is particularly meaningful to study fine particle flotation. QIU et al [8] has indicated that fine mineral particles, having high surface area and low mass, can lead to a low probability of collision and adhesion between mineral particles and air bubbles in fine particle flotation. There have already been two approaches to attempting to improve the recovery of mineral fines: to reduce bubble size and to increase particle size of floated fraction. Micro-bubble flotation has mainly been regarded as a representation of the former method [9-12]. The latter has referred to the field of hydrophobic flocculation flotation, which is considered as an effective process to deal with fine minerals, so as to form large-size clusters or flocculants [13,14]. LIU and PENG [15] demonstrated that satisfactory flotation results can be obtained by using an effective collector like styryl phosphonic acid during rutile ores processing. Numerous works by SONG et al [16-18] have reported studies on the hydrophobic flocculation of galena and sphalerite fines. They indicated that amyl xanthate as a relative short hydrocarbon chain reagent can strongly induce hydrophobic flocculation of mineral fines by kinetic energy input and showed this flocculation flotation method has increased the recovery by over 60% compared with the conventional flotation. Consequently, hydrophobic flocculation technology has been recognized as one of the most suitable methods for fine particle flotation.{刘新灿, 1991 #25}
There have also been a great number of reports on other fine mineral particles using hydrophobic flocculation technology. Hematite fines, for instance, have been induced by anionic temperature-responsive polymer [19], talc fines by kerosene [20], marmatite fines by butyl xanthate and ammonium dibutyl dithiophosphate [21] and coal fines by polyethylene oxide [22]. Little attention, however, has been paid to the hydrophobic flocculation flotation of rutile fines in the presence of flotation regents.
In order to achieve a better understanding of natural rutile fines processing, the hydrophobic flocculation flotation of rutile fines in the presence of styryl phosphonic acid were designed and studied by using flotation tests, zeta-potential measurement, optical microscope observation, laser-based particle size analysis, adsorption measurements and DLVO theory. The aim is to characterize the behavior of the flotation and hydrophobic flocculation of rutile fines affected by several parameters, namely the SPA concentration, pH, strength of shear force including stirring speed and stirring time.
2 Experimental
2.1 Minerals and chemical reagents
Rutile ores used in all experiments were obtained from Zaoyang, Hubei Province, China. Samples were purified several times by physical methods, such as gravity concentration, high gradient magnetic separation and several classifications. Rutile products less than 0.038 mm were first obtained by grinding in a vibrating cup mill. The above samples were continued to be ground with a porcelain mill. Finally, rutile particles less than 0.013 mm were obtained by 800-mesh sieve and stored in sealed glass bottles for use next time. The rutile chosen was of very high quality and its purity was >93%. The XRD diffraction pattern and multi-element analysis are presented in Fig. 1 and listed in Table 1, respectively.

Fig. 1 XRD pattern of rutile
Table 1 Multi-element analysis result of single minerals (mass fraction, %)

SPA was used as a collector, and it was an industrial grade product from a chemical factory in Changzhou, China. Sodium hydroxide and hydrochloric acid purchased from Tianheng Chemical Co., Ltd. were used to adjust pH value. The distilled water was used in the all experiments unless otherwise stated.
2.2 Flotation tests
The purified mineral particles ((2.000±0.002) g) were placed in a hitch groove cell whose effective volume is approximately 40 mL. The stirring speed of this apparatus was set at 1800 r/min in all experiments unless otherwise stated. Then, the pH value was adjusted to a given value with NaOH and HCl. After this, the collector was added into the solution. The conditioning time for the collector was 5 min and for the pH adjustor was 3 min. The flotation work was finished in 3 min. The concentrates and tailings were weighed after a series of operations like filtration and drying, and recovery was finally calculated. The detailed flotation flowchart of rutile is shown in Fig. 2.
2.3 Zeta-potential measurement
Zeta-potential measurement with a JS94H instrument was carried out on rutile before and after collector addition. The suspension was prepared by adding 20 mg of pure rutile into 40 mL of distilled water in 100 mL beaker, which contained 1×10-3 mol/L KCl as a supporting electrolyte. The prepared suspension was fully stirred with a magnetic stirrer for 15 min before zeta-potential was measured. The measuring range was limited from pH 2 to 11 in this work. These results are the average of at least three full repeating experiments.

Fig. 2 Flotation flowchart of rutile
2.4 Optical microscope observation
The optical microscope observation was carried out on OLYMPUS CX31 instrument to observe the state of particles before and after the collector addition in aqueous suspension. In each test, the prepared condition was similar to that in the flotation tests. A very small amount of suspension liquid was dropped onto the microslide that was moved to the microscope to observe after natural drying.
2.5 Laser-based particle size analysis
The laser-based particle size analysis was carried out with Master-size 2000 instrument (Malvern/England) to determine the size distributions of dispersed and flocculated rutile particles. It can directly reflect the flocculation behaviors between mineral particle and reagents. For protecting flocculation from undermining in the measurement operation, ultrasonic was not applied to the suspensions in all tests.
2.6 Adsorption measurements
Adsorption measurements were employed to investigate the change before and after chemical reactions. The UV-visible spectrophotometer of TU-1810 type, which was made in Beijing Purkinje General Instrument Co., Ltd., was used in this work. A detailed description of this instrument can be found elsewhere [23-25]. The adsorbed amount of SPA was calculated from the concentration difference between the initial and concentrate solution.
3 Results and discussion
3.1 Flotation studies
3.1.1 Effect of pH on rutile flotation
The flotation recovery of fine rutile as a function of pH using 1000 mg/L SPA as a collector is shown in Fig. 3. The result indicated that the floatability of rutile decreased as pH increased. The reason for this was that low pH can promote the formation of the phosphonate acid radical groups. And these groups were the main functional groups for adsorbing on rutile surface. Flotation recovery was up to 91% at pH 1.8 and was the highest in all experiments. Thus, SPA exhibited a strong collecting ability for rutile fines in single mineral flotation test. The results were in good agreement with the results reported by BULATOVIC and WYSLOUZIL [26]. From these flotation results, it can be concluded that SPA might have been a good floc regent for the hydrophobic flocculation of rutile fines.

Fig. 3 Relationship between mineral recovery and pH of rutile fines (SPA: 1000 mg/L)
3.1.2 Effect of SPA dosage on rutile flotation
Figure 4 shows the effect of SPA dosage on the floatability of rutile fines at pH 1.8. As SPA dosage increased, the flotation recovery of rutile promoted clearly and quickly. The maximum recovery of 90% was obtained at 1000 mg/L SPA and pH 1.8. When SPA concentration was >1000 mg/L, less than 90% of rutile fines were floated. The probable explanation was that the adsorption quantity of SPA on mineral surface came to a saturation. The recovery, thus, would not increase if the SPA dosage was excessive. At the same time, it might have a bad effect on the hydrophobic flocculation of rutile fines. Above all, the collector concentration played a key role and needed to be fixed at 1000 mg/L in such a system.

Fig. 4 Relationship between dosage of SPA and recovery of rutile fines (pH=1.8)
3.1.3 Effect of shear force on rutile flotation
Promoting collision and adhesion between fine particles and styryl phosphonic acid, the kinetic energy input provided, to a large extent, mechanical shear force in a stirred groove for the hydrophobic flocculation of fine rutile [27] {Yoon, 1996 #30}. The stirring speed in a mixed system would be mainly determined by stirring revolution, if the impeller, groove, slurry volume, and solid concentration are fixed at a constant [28]. And the magnitude of kinetic energy input is managed by stirring duration with the stirring strength fixed [18]. In this work, the effect of stirring speed and stirring time was investigated to explain how they influenced the hydrophobic flocculation floatability of rutile fines.
The effect of the shear force on the flocculation flotation of fine rutile was studied by single-mineral flotation. Figures 4 and 5 respectively illustrate the effect of stirring strength and stirring time for the flotation recovery of rutile fines induced by styryl phosphonic acid at pH 1.8 and SPA 1000 mg/L. From Fig. 4, it could be known that the best flotation recovery of 90% was at a stirring speed of 1800 r/min, and that higher stirring speed was helpful for the flocculation flotation of rutile in the whole dosage level. Thus, the results proved that kinetic energy input was beneficial to the flocculation flotation of mineral fines when SPA was used as a collector.

Fig. 5 Relationship between stirring time and recovery of rutile fines (pH=1.8 and 1000 mg/L SPA)
Figure 5 showed that the floatability of rutile clearly increased with the increase of the stirring time in the early stage. The flotation recovery reached the maximum at around 15 min, then flattened out for a time and decreased immediately after 18 min. The flocculation flotation recovery could not be enhanced and might sometimes decrease by further prolonging stirring time. The reason for the decrease in the flotation recovery after 18 min might be attributed to the destruction of the formed flocculant. Thus, controlling stirring time in an appropriate range has been a vital factor for enhancing the recovery of rutile fines.
Therefore, these results indicated that the effect of shear force was to improve the flocculation floatability of rutile fines in the system. Special attention is paid on the shear force controlled at an optimum value to obtain the best results.
3.2 Surface charge effect
The zeta-potential of rutile measured as a function of pH in the absence and presence of SPA is shown in Fig. 6. For the sake of maintaining ions strength and the double layer thickness at a constant level, KCl concentration was fixed at 1×10-3 mol/L in all experiments. The iso-electric point (IEP) of rutile was approximately 4.4 in the presence of KCl solution from experimental results, which was in good agreement with the result reported by GRAHAM and MADELEY [29]. The IEP disappeared and the value of zeta-potential became more negative with the addition of phosphonic ion. The reason for this phenomenon was the chemisorption during this process. The similar result was found in other studies [30,31]. The interaction mechanism between SPA regent and rutile particles, therefore, was the chemical adsorption rather than physical adsorption. The result was in good agreement with Ref. [32]. Based on precious researches [33,34], it can be known that the chemisorption mainly occurs in the Stern-plane, then leading to a more negative zeta-potential value.

Fig. 6 Relationship between zeta-potential of minerals and pH with or without SPA
Therefore, it could be speculated from Fig. 6 that the adsorption ability of styryl phosphonic acid on rutile particles was approximately at a constant value under acidic condition, and that the capability decreased as pH increased and then achieved to a minimum value in an alkaline solution.
3.3 Optical microscope observation of rutile fines
Figure 7 presents flocculation images of the rutile fines with different concentrations of styryl phosphonic acid. It can obviously be known from Fig. 7 that the flocculation phenomenon occurred between particles under the addition of floc reagent. The degree of the suspended particulate flocculation was strongly increased as SPA concentration was increased, which indicated that high SPA concentration could promote hydrophobic flocculation flotation of fine rutile. Otherwise, it could also be found that the best hydrophobic flocculation appeared under 1000 mg/L SPA condition.
3.4 Changes of apparent grain size

Fig. 7 Microscope images of rutile fines at pH=1.8

Fig. 8 Particle size distributions of rutile with different stirring speeds at pH=1.8
To quantitatively observe the change of floc size between particles, Fig. 8 shows the size distributions of rutile as a function of SPA concentration with different stirring speed at pH 1.8. It could be known from Fig. 8(a) that floc size had a different variation trend in different conditions, and the formation of the hydrophobic flocculate increased the size of rutile particles. The result showed that 50% volumetric diameters of rutile respectively increased from 10.45 to 12.45 and 14.55 μm under the conditions as follows: (1) the SPA concentration was 1000 mg/L and stirring speed was 1600 r/min; (2) SPA concentration was 1000 mg/L and stirring speed was 1800 r/min. And according to Fig. 8(b), the hydrophobic flocculate formation at two different stirring speeds led to the increase of volumetric mean diameters (D[4,3]) from 12.5 to 15.89 and to 20.76 μm at a SPA of 1000 mg/L.
It can also be obtained from Fig. 8 that rutile had the volumetric diameters (d90) of 33.59 and 42.13 μm after 500 and 1000 mg/L SPA was added, respectively. The volumetric mean diameters D[4,3] increased to 16.70 and to 20.76 μm, respectively. The d90 and D[4,3], however, decreased to 36.26 and 19.05 μm, respectively, when SPA concentration was no less than 1250 mg/L. Above all, the results of apparent grain size showed the same tendency with previous studies like flotation tests, and optical microscope observation.
3.5 Adsorption measurements on rutile surface
Figure 9 indicates the adsorption quantity of SPA on rutile surface with the initial concentration of reagent at different stirring speeds and pH 1.8. It was noted that the adsorption quantity was obviously higher at 1800 r/min than that at 1600 r/min in the whole range. It could be seen from Fig. 9 that adsorption quantity of SPA on minerals surface had an ascending tendency at a stirring speed of 1600 r/min with the increase of SPA dosage, which showed remarkable consistency with single- mineral experiments.

Fig. 9 Adsorption quantity of SPA on minerals surface as function of initial concentration (pH=1.8)
3.6 Theoretical calculation of inter-particle interactions
In order to accurately investigate the effect of hydrophobic flocculation between fine particles and reagent, two theories were given. The first theory used to explain the interactions involved in inter-particle adhesion is the DLVO theory, which is suitable for the aqueous solution. This theory considers that the total energy of adhesion is the result of van der Waals attractive forces and repulsive interactions. The second theory, the extended DLVO theory (EDLVO theory), is applied in the aqueous medium. This extended theory considers that the total free energy of interaction between two material surfaces is the sum of van der Waals (LW) forces, electrical double layer interactions and hydrophobic interaction in an aqueous medium [35-39].
3.6.1 Van der Waals interaction between particles
The van der Waals attractive forces VW can be determined by [8,40]:
 (1)
(1)
where H is the interaction distance among particles (nm); A is the Hamaker constant (6.34×10-20 J); R1 and R2 are the radii of particles (6 μm). From Eq. (1), it can be known that the value of VW is negative, which indicates the laterally attractive particle-particle interaction.
3.6.2 Electrostatic interaction between particles
The electrostatic double layer interactions VE can be obtained from [8,40]:

 (2)
(2)
where φ0 represents the stern potential of the particle (25.6 mV); k-1 is the Debye length (k=0.104); εa is the absolutely dielectric constant of the medium (6.950×10-10 C-2·J-1·m-1).
From Eq. (2), it can be seen that the value of VW is positive, which shows the particle-particle repulsive interaction.
3.6.3 Hydrophobic interaction between particles
The electrostatic double layer interactions VH can be determined by [8,40]

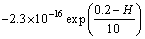 (3)
(3)
where h0 is the attenuation length (10 nm); H0 is the contact distance between particles and bubbles (0.2 nm); VH0 is the energy constant of hydrophobic interaction (-1.221×10-9 J/m2)
Equation (3) illustrates that the value of hydrophobic interactions VH is negative, which shows strongly attractive interaction of particle-bubble-particle. It can be known that the value of VH is about one order of magnitude higher than that of VE and VW from Eq. (1), Eq. (2) and Eq. (3).
Based on the DLVO theory and the EDLVO theory, the total energy of interaction  and
and 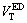 can be respectively expressed by Eqs. (4) and (5):
can be respectively expressed by Eqs. (4) and (5):
 (4)
(4)
 (5)
(5)
According to Eq. (1), Eq. (2), Eq. (4) and Eq. (5), the interaction potential-energy curve between fine rutile and SPA molecule can be obtained, as shown in Fig. 10. The value of VE was positive, which showed the repulsive interaction of particle-particle. The particle- particle interaction would become mutual attraction if VW was negative. The total interaction energy of rutile performed as mutual exclusion in aqueous solution, which provided a good explanation for existence of a small amount of cluster in Fig. 7(a). This energy, however, would be manifested as a strongly mutual attraction with the addition of SPA and it increased as the interaction distance decreased between particles. The value of theoretical calculation was consistent with the experimental data.

Fig. 10 Interaction energy of rutile between particles as function of inter-particle interaction distance
In order to more clearly illustrate the hydrophobic flocculation flotation of rutile fines in the presence of styryl phosphonic acid, an interactional model can be proposed, as shown in Fig. 11. It was obtained from the optical microscope observation that rutile particle had a good dispersion ability in aqueous solution. With the addition of SPA and the kinetic energy input, nevertheless, the dispersed particle began to flocculate and then the hydrophobic flocculate formed onto the rutile surface. The laser-based particle size analysis proved that the formation of flocs could increase the particle size. Furthermore, the different kinetic energy input (shear force) would be able to cause different floc flotation behaviors. The low shear force could only increase the size of part particles and caused lower recovery; while the high shear force could increase the size of most rutile particles and improved recovery. As a consequence, in order to improve hydrophobic flocculation floatability and flotation recovery of rutile fines, it is quite necessary to ensure appropriate kinetic energy input and proper reagent concentration.

Fig. 11 Model for interpreting interaction between particle and SPA
4 Conclusions
1) The rutile fines can be flocculated by styryl phosphonic acid (SPA), and pH, shear force, stirring time played significant roles.
2) In water solution, the total interaction energy of rutile among particles overall performed as mutual exclusion. With SPA addition, however, this energy would be manifested as strongly mutual attraction.
3) The appearance of flocculant was conducive to the floc flotation of rutile fines. And chemical adsorption was the primary reason for this.
4) The appropriate shear force could not only promote the mutual interaction between SPA and mineral particle, but also increase the size of flocculation products.
References
[1] ZIERDEN M R, VALENTINE A M. Contemplating a role for titanium in organisms [J]. Metallomics, 2016, 8(1): 9-16.
[2] YE Xiao-xin, TANG Guo-yi. Effect of coupling asynchronous acoustoelectric effects on the corrosion behavior, microhardness and biocompatibility of biomedical titanium alloy strips [J]. Journal of Materials Science: Materials in Medicine, 2015, 26(1): 1-15.
[3] LUTJERING G, WILLIAMS J C. Titanium [M]. Heidelberg: Springer Berlin, 2007.
[4] LI Zheng-yang, CAI Zhen-bing, WU Yan-ping, ZHU Min-hao. Effect of nitrogen ion implantation dose on torsional fretting wear behavior of titanium and its alloy [J]. Transactions of Nonferrous Metals Society of China, 2017, 27(2): 324-335.
[5] SCANLON D O, DUNNILL C W, BUCKERIDGE J, SHEVLIN S A. Band alignment of rutile and anatase TiO2 [J]. Nature Materials, 2013, 12(9): 798-801.
[6] JOHNSON N W. Liberated 0-10 μm particles from sulphide ores, their production and separation—Recent developments and future needs [J]. Minerals Engineering, 2006, 19(6): 666-674.
[7] XU Dan-feng, AMTOV I, GRANO S R. Quantifying rheological and fine particle attachment contributions to coarse particle recovery in flotation [J]. Minerals Engineering, 2012, 39(1): 89-98.
[8] QIU Guan-zhou, HU Yue-hua, WANG Dian-zuo. The interaction between the particles and fine particle flotation [M]. Changsha: Central South University of Technology Press, 1993. (in Chinese)
[9] LI Xiao-bing, XU Hong-xiang, LIU Jiong-tian, ZHANG Jian, LI Jing, GUI Zhao-long. Cyclonic state micro-bubble flotation column in oil-in-water emulsion separation [J]. Separation & Purification Technology, 2016, 165(1): 101-106.
[10] YAN Xiao-kang, LIU Jiong-tian, CAO Yi-jun, WANG Li-jun. A single-phase turbulent flow numerical simulation of a cyclonic-static micro bubble flotation column [J]. International Journal of Mining Science & Technology, 2012, 22(1): 95-100.
[11] LI Guo-sheng, LIU Jiong-tian, CAO Yi-jun, WANG Da-peng. Effect of a cyclonic flotation column on the separation of magnesium from phosphate ore [J]. Mining Science and Technology (China), 2011, 21(5): 647-650.
[12] TAN Xin, HE Fa-yu, SHANG Yan-bo, YIN Wan-zhong. Flotation behavior and adsorption mechanism of (1-hydroxy-2-methyl-2- octenyl) phosphonic acid to cassiterite [J]. Transactions of Nonferrous Metals Society of China, 2016, 26(9): 2469-2478.
[13] ZHANG Ting, QIN Wen-qing. Floc flotation of jamesonite fines in aqueous suspensions induced by ammonium dibutyl dithiophosphate [J]. Journal of Central South University, 2015, 22(4): 1232-1240.
[14] FORBES E. Shear, selective and temperature responsive flocculation: A comparison of fine particle flotation techniques [J]. International Journal of Mineral Processing, 2011, 99(1-4): 1-10.
[15] LIU Qi, PENG Yong-jun. The development of a composite collector for the flotation of rutile [J]. Minerals Engineering, 1999, 12(12): 1419-1430.
[16] SONG Shao-xian, LOPEZ-VALDIVIESO A, REYES-BAHENA J L, BERMEJO-PEREZ H I. Hydrophobic flocculation of sphalerite fines in aqueous suspensions induced by ethyl and amyl xanthates [J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2001, 181(1): 159-169.
[17] SONG Shao-xian, LOPEZ-VALDIVIESO A, REYES-BAHENA J L, LARA-VALENZUELA C. Floc flotation of galena and sphalerite fines [J]. Minerals Engineering, 2001, 14(1): 87-98.
[18] SONG Shao-xian, LOPEZ-VALDIVIESO A, REYES-BAHENA J L, BERMEJO-PEREZ H I, TRASS O. Hydrophobic flocculation of galena fines in aqueous suspensions [J]. Journal of Colloid and Interface Science, 2000, 227(2): 272-281.
[19] NG Wei-Sung, SONSIE R, FORBES E, FRANKS G V. Flocculation/ flotation of hematite fines with anionic temperature-responsive polymer acting as a selective flocculant and collector [J]. Minerals Engineering, 2015, 77(1): 64-71.
[20] OZKAN A, DUDNIK V, ESMELI K. Hydrophobic flocculation of talc with kerosene and effects of anionic surfactants [J]. Particulate Science and Technology, 2016, 34(2): 235-240.
[21] ZHANG Ting, QIN Wen-qing, YANG Cong-ren, HUANG Shui-peng. Floc flotation of marmatite fines in aqueous suspensions induced by butyl xanthate and ammonium dibutyl dithiophosphate [J]. Transactions of Nonferrous Metals Society of China, 2014, 24(5): 1578-1586.
[22] LIANG Long, TAN Jia-kun, LI Zhi-yuan, PENG Yao-li, XIE Guang-yuan. Coal flotation improvement through hydrophobic flocculation induced by polyethylene oxide [J]. International Journal of Coal Preparation and Utilization, 2016, 36(3): 139-150.
[23] LIU Run-qing, SUN Wei, HU Yue-hua, WANG Dian-zuo. Surface chemical study of the selective separation of chalcopyrite and marmatite [J]. Miningence & Technology, 2010, 20(4): 542-545.
[24] XIAO Wei, FANG Chao-jun, WANG Jun. The role of EDTA on rutile flotation using Al3+ ions as an activator [J]. Rsc Advances, 2018, 8(9): 4872-4880.
[25] XIAO Wei, CAO Pan, LIANG Qian-nan, WANG Jun. Adsorption behavior and mechanism of Bi(III) ions on rutile–water interface in the presence of nonyl hydroxamic acid [J]. Transactions of Nonferrous Metals Society of China, 2018, 28(2): 348-355.
[26] BULATOVIC S, WYSLOUZIL D M. Process development for treatment of complex perovskite, ilmenite and rutile ores [J]. Minerals Engineering, 1999, 12(12): 1407-1417.
[27] LU Shou-ci, WENG Da. Principles and applications of inter-face separation [M]. Beijing: Metallurgical Industry Press, 1992. (in Chinese)
[28] KOH P T L, ANDREWS J R G, UHLHERR P H T. Floc-size distribution of scheelite treated by shear-flocculation [J]. International Journal of Mineral Processing, 1986, 17(1-2): 45-65.
[29] GRAHAM K, MADELEY J D. Relation between the zeta potential of rutile and its flotation with sodium dodecyl sulphate [J]. Journal of Applied Chemistry, 2007, 12(11): 485-489.
[30] LOPEZ-VALDIVIESO A, ROBLEDO-CABRERA A, URIBE- SALAS A. Flotation of celestite with the anionic collector sodium dodecyl sulfate. Effect of carbonate ions [J]. International Journal of Mineral Processing, 2000, 60(2): 79-90.
[31] HAN K N, HEALY T W, FUERSTENAU D W. The mechanism of adsorption of fatty acids and other surfactants at the oxide-water interface [J]. Journal of Colloid and Interface Science, 1973, 44(3): 407-414.
[32] DU Yan. Flotation separation of rutile using alkyl amine dimethyl phosphonic acid [J]. Mining and Metallurgical Engineering, 1993, 13(2): 34-37. (in Chinese)
[33] HIEMENZ P C, RAJAGOPALAN R. Principles of colloid and surface chemistry [M]. Third edition. New York: CRC Press, 1997.
[34] RIDEAL E, DAVIES J. Interfacial phenomena [M]. London: Academic Press, 1963.
[35] ZHANG Zhi-jun, LIU Jiong-tian, FENG Li, WANG Yong-tian, ZHANG Ming-qing. Calculation of critical hardness of coal slime water system based on DLVO theory [J]. Journal of China University of Mining & Technology, 2014, 43(1): 120-125. (in Chinese)
[36] PINERES J, BARRAZA J. Energy barrier of aggregates coal particle–bubble through the extended DLVO theory [J]. International Journal of Mineral Processing, 2011, 100(1): 14-20.
[37] AZEREDO J, VISSER J, OLIVEIRA R. Exopolymers in bacterial adhesion: Interpretation in terms of DLVO and XDLVO theories [J]. Colloids and Surfaces B: Biointerfaces, 1999, 14(1): 141-148.
[38] YOON Roe-Hoan, MAO Lai-qun. Application of extended DLVO theory, IV: Derivation of flotation rate equation from first principles [J]. Journal of Colloid & Interface Science, 1996, 181(2): 613-626.
[39] HU Yue-hua, QIU Guan-zhou, WANG Dian-zuo. Extended DLVO theory and its applications in flotation of fine particles [J]. Journal of Central South Institute of Mining and Metallurgy, 1994, 25(3): 310-314. (in Chinese)
[40] ISRAELACHBILI J N, JACOB N. Intermolecular and Surface Forces [M]. Third edition. USA: Academic Press, 2011.
黄小涛1,2,肖 巍1,2,赵红波1,2,曹 攀1,2,胡奇秀1,2,覃文庆1,2,张雁生1,2,邱冠周1,2,王 军1,2
1. 中南大学 资源加工与生物工程学院,长沙 410083;
2. 中南大学 生物冶金教育部重点实验室,长沙 410083
摘 要:通过浮选试验、动电位测试、显微镜观测、激光粒度分析、吸附量测定和DLVO理论研究细粒金红石在苯乙烯膦酸(SPA)体系中的絮团浮选行为。单矿物浮选试验结果表明,苯乙烯膦酸对细粒金红石的絮团浮选具有良好的诱导作用;同时,溶液pH值、剪切力(搅拌速率)和搅拌时间均对絮团浮选效果有一定影响。动电位测试发现,随着SPA的加入,等电点及电位均负向移动,表明矿物与药剂之间主要发生化学吸附。激光粒度分析表明,在搅拌速度为1800 r/min和1000 mg/L SPA时,金红石颗粒的尺寸最大。此外,通过显微镜观测和浮选试验证明絮团的产生有利于细粒金红石的浮选。综上可得,SPA通过化学吸附作用能有效诱导细粒金红石的疏水絮团并增大其颗粒尺寸。最后,通过DLVO理论计算进一步验证SPA 与金红石颗粒之间主要发生化学吸附作用,进而促进絮团产物的形成。
关键词:疏水絮团浮选;细粒金红石;苯乙烯膦酸;DLVO理论
(Edited by Bing YANG)
Foundation item: Projects (51474254, 51774332, 51320105006) supported by the National Natural Science Foundation of China; Project (NCET-13-0595) supported by the Program for New Century Excellent Talents in University, China; Projects (2017zzts579, 2017zzts379) supported by the Fundamental Research Funds for the Central Universities of China
Corresponding author: Jun WANG; Tel: +86-731-88876557; E-mail: wjwq2000@126.com
DOI: 10.1016/S1003-6326(18)64781-8

