
Recycle and synthesis of LiCoO2 from incisors bound of Li-ion batteries
LIU Yun-jian(刘云建), HU Qi-yang(胡启阳), LI Xin-hai(李新海),
WANG Zhi-xing(王志兴), GUO Hua-jun(郭华军)
School of Metallurgical Science and Engineering, Central South University, Changsha 410083, China
Received 19 October 2005; accepted 23 February 2006
Abstract:A new LiCoO2 recovery technology of Li-ion battery was studied. LiCoO2 was initially separated from the Al foil with dimethyl acetamide(DMAC), and then the polyvinylidene fluoride(PVDF) and carbon powders in the active material were eliminated by high temperature calcining. The content of the elements in the recovered powder was analyzed. The structure and morphology of the resulted samples were observed by XRD and SEM. Then the Li2CO3 was added in the recycled powder to adjust the Li/Co molar ratio to 1. The new LiCoO2 was synthesized by calcining at 850 ℃ for 12 h in air. The well-crystallized single phase LiCoO2 without Co3O4 phase was obtained. The recycle-synthesized LiCoO2 powders have good characteristics as a cathode active material in terms of charge-discharge capacity and cycling performance.
Key words:Li-ion battery; direct recovery; LiCoO2; synthesis; electrochemical performance
1 Introduction
Since the Sony Energytec unveiled the first commercial Li-ion cell[1], the Li-ion battery has become the most attractive energy source for portable electronic products, such as mobile phone, notebook computers. The market for Li-ion battery has expanded rapidly because of the increase in demand for mobile electronics. It is reported that the total sales of the cell in 2002 have reached 6.5 billion dollars, and are expected to be more than 10 billion dollars in 2005[2]. The layered LiCoO2 is used most for commercial products at present. It demands large amount of Co to meet the market. It is very important to recycle the Co in the Li-ion battery, because Co is a rare element, and is not friendly to environment. The spent Li-ion batteries and its incisors bound come out from the made process can give rise to the environmental poisoning. In other hand recycling the Co can also bring economical interests.
At present, many researchers have studied how to recycle the spent Li-ion batteries. It has been reported[3] that cobalt ions extracted from waste LiCoO2 by using a nitric acid solution leaching, were potentostatically transformed into cobalt hydroxide on a titanium electrode and cobalt oxide was then obtained via a dehydration procedure. ZHANG et al[4] reported the recycle of the metal values such as cobalt and lithium from the spent Li-ion secondary batteries. LAIN[5] recycled metal values from the cell using AEA technology. They just recycled metal values from the waste batteries. But LEE et al[6] and CONTESTAILE et al[7] not only recycled the metal values but also prepared new LiCoO2 just using the recycled metal values, and showed good results.
Many incisors bound of LiCoO2 is produced in the process of Li-ion battery manufacturing. It is waste to discard the incisors bound. In this paper, a new LiCoO2 recovery technology from the incisors bound of Li-ion battery is studied. After recycle process, there is little Co3O4 in the cycled LiCoO2. The final LiCoO2 powder is synthesized at high temperature with Li2CO3. The electrochemical performances of the synthesized LiCoO2 has also been studied.
2 Experimental
At first, a certain incisors bound of Li-ion battery was dipped in DMAC. Several hours later the aluminum foil was picked out. And then the solution was filtrated after several hours deposit. The cathode material without aluminum was gained after filtrating. Then the cathode material was heated at 120 ℃ in air for 12 h. The solvent DMAC was vaporized during the heating process. The powder was gained after milling. The dried powder was firstly heated at 450 ℃ in air for 2 h, then heated at 600 ℃ for 5 h. To prevent the agglomeration, the powder should be filled loosely. Then the recovered powder was gained after washing the heated powder several times with distilled water and drying at 80 ℃ for several hours. The structure of the recycled powders was identified with XRD technique. The content of the Co was analysed by titrating, and the element Li by atomic absorption spectrometry.
The recycled powders containing LiCoO2, Co3O4 and Li2CO3 were used as starting materials. The ratio of Li/Co was adjusted to 1 by adding a certain amount of Li2CO3. The final LiCoO2 was synthesized by calcaning at 850 ℃for 12 h in air, and cooled to the room temperature in a tube-furnace. The heat-treated products were ground in an agate mortar. The structure and morphology of the obtained samples were measured with XRD and SEM, respectively.
The powder X-ray diffraction (XRD, Rint-2000, Rigaku) using Cu Kα radiation was employed to identify the crystalline phase of the synthesized materials. The particle size and morphology of the LiCoO2 powders were measured by scanning electron microscope (JEOL, JSM-5600LV) with an accelerating voltage of 20 kV.
The electrochemical characterization was performed using CR2025 coin-type cell. For cathode fabrication, the prepared powders were mixed with 10%(mass fraction) carbon black and 10% polyvinylidene fluoride in N-methyl pyrrolidinone to obtain slurry. And then, the blended slurries were pasted onto an aluminum current collector, and the electrode was dried at 100 ℃ for 12 h in vacuum. The test cell consisted of the cathode and lithium foil anode separated by a porous polypropylene film, cellgard 2 300 as the separator and 1 mol/L LiPF6 in EC:EMC:DMC (1:1:1 in volume) as the electrolyte. The assembly of the cells was carried out in a dry Ar-filled glove box. The capacity measurements and cycling tests of the coin-type cells were carried out between 3.0 V and 4.2 V at 0.2C rate at 25 ℃.
3 Results and discussion 3.1 Recycle process
The PVDF used in the cathode as the binder can be dissolved in organic solvent such as N-methyl pyrrolidinone(NMP), DMAC. The solubility of PVDF in the NMP or DMAC is about 10%. In this study DMAC was chosen as solvent in the experiment for the economic consideration. The solution will become more viscid when the PVDF is dissolved in the solvent DMAC. So the ratio of solid and solution should be controlled. The ratio was controlled between 1:4 and 1:5 in our experiment. The result shows that the incisors bound was easily dissolved in the solvent. The cathode material can be easily separated from the aluminum foil, and can be easily filtrated from the solvent. The boiling point of DMAC is 165 ℃, so DMAC can be evaporated by heating at 120 ℃ for 12 h. There are cathode material LiCoO2, PVDF and carbon powders in the recycled powders.
The cathode of Li-ion battery is made up of the LiCoO2, PVDF and carbon powders. So LiCoO2 can be gained after eliminating PVDF and carbon powers.
The sample was heated at 450 ℃ for 2 h in our experiment. The heated and unheated samples were characterized by IR spectrum (Fig.1). In Fig.1(a) the organic compound can be found at the wavenumber of 2 910, 1 400 and 1 183 cm-1 obviously in the unheated powders. But no organic compound exits in the heated powders as shown in Fig.1(b). So it can be concluded that PVDF is decomposed after heating at 450 ℃ for 2 h.
The XRD pattern of the recycled powders after heating is shown in Fig.2. The peaks of Co3O4 at 31.3?, 36.8? and CoO at 42.4? were observed. The pure LiCoO2 added 5% PVDF and 5% carbon powder respectively were heated to identify the presence of Co3O4 and CoO. The two samples were tested with XRD (Fig.3). In Fig.3 the peaks of Co3O4 and CoO do not exit in the carbon added LiCoO2 powders. But the peak of Co3O4 is observed in the sample added PVDF. So it can be concluded that the appearance of Co3O4 is due to PVDF addition.
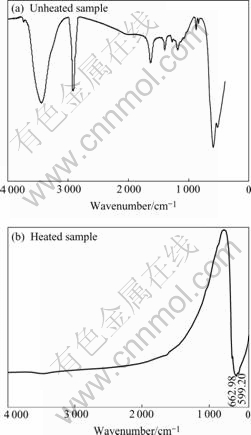
Fig.1 IR spectra of samples
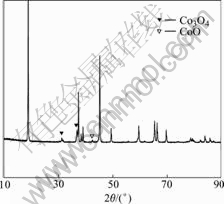
Fig.2 XRD pattern of heated sample
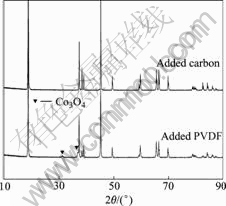
Fig.3 XRD patterns of LiCoO2 reacting with carbon and PVDF
Because PVDF can give out HF when heating, HF reacts with LiCoO2 to produce HCoO2, and HCoO2 is not steady at high temperature, it will decompose to Co3O4.
The carbon powder is easy to eliminate because it is very easy to oxidize by O2 at high temperature such as 600℃ in air. So when the powder was heated at 600 ℃ for 5 h, the carbon powder should be eliminated.
The content of the elements in the recycled powders was analysed. The result is shown in Table 1. From Table 1, the contents of C and F are very low, and the Li/Co molar ratio is 0.914.
Table 1 Content of elements in recycled LiCoO2 (mass fraction, %)

Fig.4 shows the XRD pattern of the recovered sample after heating. In Fig.4 the peak of CoO appearing in Fig.2 disappears. This is because the CoO has been oxidized to Co3O4 by O2 at high temperature. So there is only little Co3O4 in the recycled LiCoO2 powders.
3.2 Further treatment of LiCoO2
The final LiCoO2 was obtained by adding a certain amount of Li2CO3 in the recycled LiCoO2 and calcining
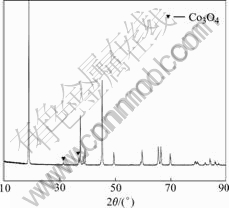
Fig.4 XRD pattern of recycled sample
at 850 ℃ for 12 h. The structure of HT-LiCoO2 belongs to the trigonal system (space group R3m, O3 phase) with an ideal NaFeO2 layered structure, in which Co and Li planes alternate in the ABCABC oxygen stacking[8]. Fig.5 shows the XRD pattern of the recycle-synthesized LiCoO2. From Fig.5, we can see that the spinel phase of cobalt oxide, Co3O4, belonging to the space group Fd3m, disappears, because Co3O4 reacts with Li2CO3 at high temperature. After calcined at 850 ℃ for 12 h, the well-crystallized single phase LiCoO2 can be obtained. The high intensity of the (003) peak and the clear splitting between the (006)/(012) and (018)/(110) peaks can be observed in Fig.5. The lattice parameters of the sample calcined at 850 ℃ for 12 h are a=2.817?, c=14.063 ? (c/a=4.992), which agrees with literature data for LiCoO2[9].
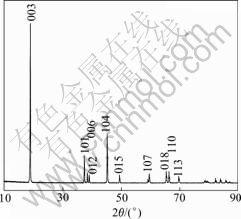
Fig.5 XRD pattern of final LiCoO2
Fig.6 shows the SEM photograph of LiCoO2 obtained. The fine particles can be observed. The size of the recovered LiCoO2 with homogenous distribution is 1-5 μm. The well-defined shape is observed for LiCoO2 calcined at 850 ℃ for 12 h.
3.3 Electrochemical properties
The coin-type cells were charge/discharged between 3.0 V and 4.2 V at 0.2C rate. The first charge-discharge curves for the recycle-synthesized LiCoO2 are shown in Fig.7. The major potential plateau around 3.9 V is shown in both charge and discharge profiles. It is one of the typical properties for the layered LiCoO2 phase, which corresponds to the reversible two-phase reactions in Li1-xCoO2 (0<x<1/4) in a topotactic manner[9-11]. Two smaller plateaus are also present at higher potentials, which correspond to the order/disorder phase transition arising at around x=0.5 in the HT-Li1-xCoO2 [12].
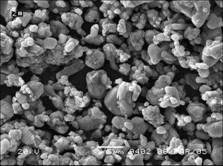
Fig.6 SEM of final LiCoO2
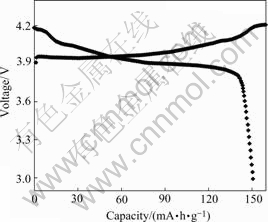
Fig.7 First charge-discharge curves for recycle-synthesized LiCoO2
The charge capacity in the first cycle is 161 mA?h/g, and the discharge capacity is 151mA?h/g. The discharge efficiency is 93.5%.
The cycle performance of the recycle-synthesized LiCoO2 is shown in Fig.8. A capacity loss is observed in
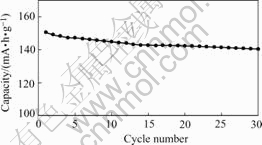
Fig.8 Cycling performance of recycle-synthesized LiCoO2
the first tenth cycles, and with the cycle number increasing, the capacity loss becomes smaller. After 30 cycles, the discharge capacity is still 141 mA?h/g, 6.3% fading compared with the values on the first discharge.
4 Conclusions
The technology of recycle and synthesis of LiCoO2 from the incisors bound of Li-ion batteries was studied. The active material containing PVDF and carbon powder can be separated from the Al foil by using DMAC as impregnant. PVDF and carbon can be eliminated by heating at 450 ℃ and 600 ℃, respectively. At last, there is only little Co3O4 in the recycled LiCoO2 powders.
The final LiCoO2 is obtained by adding a certain amount of Li2CO3 in the recycled powders and calcining at 850 ℃ for 12 h in air. The first discharge capacity of the recycle-synthesized LiCoO2 is 151 mA?h/g. After 30 cycles, the discharge capacity is still 141 mA?h/g, 6.3% fading compared with the first discharge capacity.
References
[1] BOK J S, LEE J H, LEE B K, KIM D P, RHO J S, YANG H S, HAN K S. Effects of synthetic conditions on electrochemical activity of LiCoO2 prepared from recycled cobalt compounds [J]. Solid State Ionics, 2004, 169(1-4): 139-144.
[2] KIM J, KIM B, LEE J G, CHO J, PARK B. Differential voltage analyses of high-power, lithium-ion cells(1): Technique and application [J]. J Power Sources, 2005, 139(-2): 289-294.
[3] MYONG J, JUNG Y W, LEE J Y, YONGSUG T. Cobalt oxide preparation from waste LiCoO2 by electrochemical–hydrothermal method [J]. J Power Sources, 2002, 112(2): 639-642.
[4] ZHANG P W, TOSHIRO Y, OSAMU I, SUZUKI T M, KATSUTOSHI I. Hydrometallurgical process for recovery of metal values from spent lithium-ion secondary batteries [J]. Hydro- metallurgy, 1998, 47 (2-3): 259-271.
[5] LAIN M J. Recycling of lithium ion cells and batteries [J]. J Power Sources, 2001, 97-98(1): 736-738.
[6] LEE C K, RHEE K I. Preparation of LiCoO2 from spent lithium-ion batteries [J]. J Power Sources, 2002, 109(1): 17-21.
[7] CONTESTAILE M, PANERO S, SCROSATI B. A laboratory-scale lithium-ion battery recycling process [J]. J Power Sources, 2001, 92(1-2): 65-69.
[8] ERMETE A. LiCoO2: formation, structure, lithium and oxygen nonstoichiometry, electrochemical behaviour and transport properties [J]. Solid State Ionics, 2004, 170(3-4): 159-171.
[9] AMATUCCI G G, TARASCON J M, KLEIN L C. CoO2, the end member of the LixCoO2 solid solution [J]. J Electrochem Soc, 1996, 143(4): 1114-1118.
[10] PAULSEN J M, MUELLER-NEUHAUS J R, DAHN J R. Layered LiCoO2 with a different oxygen stacking (O2 structure) as a cathode material for rechargeable lithium batteries [J]. J Electrochem Soc, 2000, 147(3): 508-516.
[11] OHZUKU T, UEDA A. Solid-state redox reactions of the LiCoO2(R3m) for 4 volt secondary lithiun cell [J]. J Electrochem Soc, 1994, 141(10): 2972-2975.
[12] REIMIERS J N, DAHN J R. Electrochemical and in situ X-ray diffraction studies of lithium intercation in LixCoO2 [J]. J Electrochem Soc, 1992, 139(7): 2091-2196.
(Edited by YUAN Sai-qian)
Corresponding author: LIU Yun-jian; Tel/Fax: +86-731-8836633; E-mail: lyjian122331@163.com