
Carbon solubility in Cu-based composite prepared by mechanical alloying
RAN Xu(冉 旭)1,2, LIU Yong-bing(刘勇兵)1, BAO Xiao-jun(包晓军)1, 3,
LIU Xue-ran(刘学然)1, AN Jian(安 健)1
1. Key Laboratory of Automobile Materials, Ministry of Education, Department of Materials Science and Engineering;
Jilin University, Changchun 130025, China;
2. Department of Materials Science and Engineering, Changchun Institute of Technology, Changchun 130012, China;
3. Earthquake Administration of Jilin Province, Changchun 130022, China
Received 20 April 2006; accepted 30 June 2006
Abstract: The powder mixture of Cu and graphite was mechanically alloyed (MA) in an oscillating type ball mill. The milling time was varied in order to investigate its influence on the microstructural evolution of mechanically alloyed powders. The phase constituent, alloying characteristics, grain size and lattice distortion of these powders were determined by X-ray diffraction, X-ray photoelectron spectroscopy, scanning electron microscopy and transmission electron microscopy. The results show that the C is confirmed to dissolve in the Cu lattice, forming solid solution of carbon in copper the lattice parameter of copper increases with carbon concentration increasing, up to a saturation value of about 4%C(mass fraction). Higher ball-mill energy is beneficial for twins and nanograin formation.
Key words: mechanical alloying; supersaturated solid solution; Cu-C system
1 Introduction
Many works have been carried out to check the possibility of replacing silver by copper in electronic contacts application, in which the stability of copper-based composites with carbon (Cu/C) was concentrated on[1-3]. By adding carbon to copper matrix it is possible to improve material’s mechanical and sliding properties. This composite is one of the most acceptable stuff used for low voltage circuit breakers and electrical brush. Carbon is considered to be insoluble in copper up to very high temperatures since its solubility does not exceed 0.02%[4]. In addition, extraordinarily small wettability of carbon by copper impedes the fabrication of the composite material. However, several reports have suggested that a metastable solid solution may be formed using unconventional non-equilibrium processing techniques including mechanical alloying(MA), radio frequency magnetron sputtering co-deposition of carbon and copper as well as ion implantation of carbon into a copper substrate. Mechanical alloying(MA) is a solid-state powder processing technique involving repeated welding, fracturing and rewelding of powder particles in a high-energy ball mill. MA has now been shown to be capable of synthesizing a variety of equilibrium and nonequilibrium alloy phases starting from blended- elemental or prealloyed powders[5-7]. The non-equilibrium phases synthesized include supersaturated solutions, metasable crystalline and quasicrystalline phases, nanostructures, and amorphous alloys[8, 9]. In this communication we report on the results of X-ray diffractometer(XRD), transmission electron microscopy(TEM) and scanning electron microscopy(SEM) examinations of the microstructure characteristics in Cu-C system by MA.
2 Experimental
The elemental materials used in this study were 99.5% pure Cu powder with a particle size range of 40-100 μm and 99.0% pure C powder with a particle size of 20-45 mm. Powders with various carbon content (2%, 4%, 6%, 8%, mass fraction) were mechanically alloyed for different times (8, 16, 24, 30 h) in an oscillating type ball mill. The hardened steel mill container (68 mm×75 mm) was charged with mixture of the elemental powders and GCr15 steel balls (d9 mm) and a rotation speed of 470 r/min was used. The ball-powder mass radio was 4∶1. The resultant powder was characterized by XRD using Cu Kα radiation, XPS, SEM and TEM. The data from X-ray diffraction allowed determination of the crystalline size and the lattice parameter. The crystallite size was estimated from least square error method using the most intense Bragg reflection Cu(111) and the lattice parameter was determined using (311), (222) and (331) copper reflections.
3 Results
Fig.1 shows the XRD patterns of Cu-4%C powders milled for different times. It is noted that before milling the reflections of both graphite (0002) and Cu can be clearly observed. As milling time is longer than 8 h the graphite peak disappears. With the increase of the mechanical alloying time, the diffraction peaks shift to lower diffraction angles, and appear to be broadened except those milled for 30 h. The change in position and shape of diffraction peak indicates something happened to the physical nature such as lattice parameter, solubility and local strain. The peak shift corresponds to the change in solubility of carbon in the copper. Generally speaking, the diffraction peaks are sharp when numerous perfect crystals with grain size of 0.2-20 μm are present and their orientations are random, whereas the diffraction peaks are broad when the grain size is smaller than 200 nm and the crystals have a large local strain. As mentioned
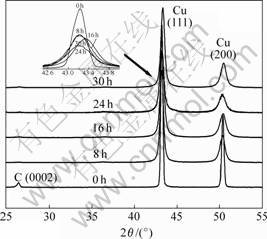
Fig.1 X-ray diffraction patterns of Cu-4%C powders mechanically alloyed for various times
above, during mechanical alloying, milling balls cause cold joints in the mixed powders and crush them numerous times, resulting in the accumulation of plastic strain and grain refinement.
Fig.2 shows the variation of lattice parameter of Cu-C alloy as a function of milling time. Three materials display the same trend with milling time. At beginning, the lattice parameter increases with milling time, and reaches a maximum value at approximately 24 h; beyond that value the lattice parameter decreases. It suggests that the lattice parameter increase is due to the diffusion of C into the Cu lattice, i.e. carbon atoms occupy interstitial positions at centers of the octahedral lattices, forming a Cu(C) solid solution. The evolution of the copper grain size with milling time is shown in Fig.3. It is confirmed that a nanocrystalline grain structure in the powder is formed during the milling process. The copper grain size decreases with increasing milling time and the carbon content in Cu-C powders. The copper grains after 30 h milling have a diameter of about 35 nm for Cu-4%C alloy, 28 nm for Cu-6%C alloy and 18 nm for Cu-8%C alloy, respectively.

Fig.2 Variations of lattice parameter with milling time

Fig.3 Variations of grain size with milling time
In order to clarify the chemical nature of C in Cu-C alloy during MA, XPS analysis was conducted. Fig.4 shows the XPS spectra fitting curve of the C1s region for Cu-4%C sample by MA. It can be seen that the carbon exhibits two kinds of bonds, i.e. C—O and C—C after 8 h milling, whereas another bond of C—Cu is formed, as the milling time is more than 8 h, indicating onset of reaction between C and Cu. As the C and Cu cannot form compound, the C will be confirmed to dissolve in the Cu lattice, forming solid solution of carbon in copper.
Fig.5 shows the microstructures of the powders of Cu-4%C mixture after different times of milling. With
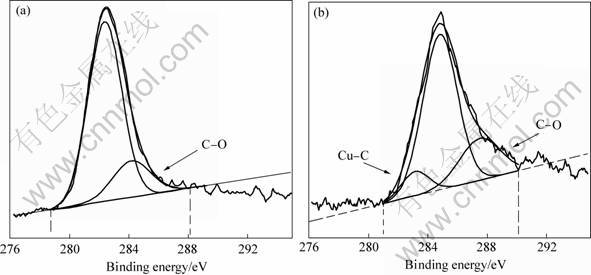
Fig.4 XPS spectra fitting curves of C1s region for Cu-4%C sample by MA: (a) MA 8 h; (b) MA 24 h
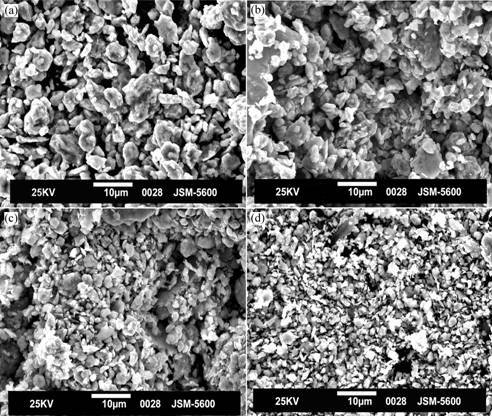
Fig.5 SEM micrographs of C/Cu powder milled for different times: (a) Cu-4%C MA 8 h; (b) Cu-4%C MA16 h (c) Cu-4%C MA24 h; (d) Cu-4%C MA30 h
increasing milling time, the originally dendritic copper powder becomes spherical in shape owing to the impact forces exerted on the powder by the grinding medium. There is a continuous decrease in the particle size of composite powders with increasing milling time. At this stage, the graphite particles appear to be segregated between the matrix powders.
The microstructure characteristic of Cu-4%C powder for a milling time of 30 h was examined by TEM as shown in Fig.6. A very fine nanocrystalline grain structure (Fig.6(a)), nanometer/micron composite particle morphology (Fig.6(b)) and mechanical twins (Fig.6(c)) coexist in one specimen. It can be inferred that the microstructure is inhomogeneous through the specimen.
4 Discussion
4.1 Supersaturated solid solution of carbon in copper
Solid solubility extensions have been achieved in many alloy systems by mechanical alloying. Solid solubility levels have been generally determined from changes in the lattice parameter values, which are calculated from shifts in peak positions in the X-ray diffraction patterns. The dependence of the copper lattice parameter on milling time shown in Fig.2 can be interpreted as dissolution of graphite into copper, which can be assumed to take place by an interstitial mechanism that carbon atomic occupy interstitial positions at centers of the octahedral lattices. This leads to an increase of the copper lattice parameter and internal strain. However, the decrease of the copper lattice parameter after 24 h is difficult to rationalize. Several hypotheses are conceivable: first, precipitation of carbon from the supersaturated solid solution may occur, but neither carbon diffraction peak in the XRD diagrams nor observation in TEM can give direct evidence for these precipitates may be so small that it is impossible to measure. Another possibility is formation of stacking faults during intense milling. Copper is known to have low stacking fault energy[10]. Hence, a high density of stacking faults can be expected. Consequently, these stacking faults may cause a peak shift opposite to that caused by the dissolved carbon in copper. Our TEM investigation proves that some mechanical twins exist in the mechanical alloyed powder. Twins can be formed by accumulation of stacking faults. Therefore, the second mechanism may be the reason for the decrease of lattice parameter. Further investigation is still needed.
4.2 Formation mechanism of composite particles
The powders obtained after MA has both small particle size and very fine grain size. The mechanism for the formation of the composite particles in this paper may be proposed as follows. In the early stages of milling, the ductile copper matrix powder particles get glomeration and then are coated with graphite. With milling continuing, these particles are cold welded, work hardened, fragmented, and subsequently become convoluted. Repeated processes of fracture and convolution applied to the composite particles result in increasing of the defect density. When the defect densities in the microstrain area reach a critical value, coarse grain is cracked into fine grain, and finally transformed into nanograin. The formed mixture powders include two kinds of particles: one is formed by separate nanograin (Fig.6(a)), the other is the mixture formed by two types of particles (Fig.6(b)). In other words, these large particles observed here are the micrometer or submicrometer particles consisted of nanograin.
4.3 Formation of mechanical twins
A face-centre-cubic (fcc) metal is considered to be difficult to deform by twinning, since it has enough slip systems to fulfill the deformation. Mechanical twins are never observed in copper at room temperature by cyclic fatigue or tensile stress. BLEWITT et al obtained twins

Fig.6 TEM micrographs of Cu-4%C powder milled for 30 h: (a) nano-particle; (b) composite particle; (c) twins
during the tensile testing of certain copper crystals at low temperatures. Since then, several workers have reported additional observations of this mode of deformation. Mechanical twin defects can be formed by plastic deformation and phase transformation in the metals and alloys. Among the available twinning mechanisms, Venable[11,12]model is most suitable to explain the formation of mechanical twins in the present study, since the deformation mode changes from slip into microtwinning in explosively deformed Cu after mechanical alloying as shown in Fig.6(c). According to Venables, the stress required to activate a twinning dislocation of length l is
nτT=S/bl+Gbl/l (1)
where n is stress concentration factor; bl is the Burgers of the Shockley partial involved in twinning; S is the stacking fault energy of the metal. On the other hand, with increasing plastic deformation the dislocation density increases to certain extent such that the critical length of the pinned dislocation u is attained. The shear stress to bow out such a dislocation is given by
τ=Gb/u (2)
where G is the rigidity modulus; b is the Burgers vector of the unit dislocation. By assuming u to be nearly 1, and comparing these two values of stress, then, the critical stress for twinning is obtained by eliminating u from Eqns.(1) and (2)
τ=Sb/[bl(nb-bl)] (3)
In the case of pure copper, if one takes the parameters in Eqns.(2) and (3), for n=1 we obtain τ=1.11 GPa. The output pressure of mechanical alloy is expressed as
pmax=gPv0.4(ρ/Eeff)0.2Eeff (4)
At last, we insert the mechanical alloying parameters into Eqn.(4), pmax=9.72 GPa is obtained. Since pmax induced by MA is larger than 1.11 GPa, twinning should be the preferred mode of deformation.
5 Conclusions
Solid solubility extensions have been achieved in C/Cu alloy systems by mechanical alloying. The lattice parameter increases with milling time, and reaches a maximum value at approximately 24 h; beyond that value lattice parameter decreases. The copper grain size decreases with increasing milling time and carbon content in Cu-C powders. The formed mixture powders include two kinds of particles: one is formed by separate nanograin; the other is the micrometer or submicrometer particles consisted of nanograins. Mechanical twin defects can be formed by plastic deformation in C/Cu alloy systems by mechanical alloying.
References
[1] IIJIMA S. Helical microtubes of graphitic carbon[J]. Nature, 1991, 354: 56-58.
[2] BERNER A, FUKS D, ELLIS D E. Formation of nano-crystalline structure at the interface in Cu-C composite[J]. Applied Surface Science, 1999, 144-145: 677-681.
[3] BERNER A, MUNDIM K C, ELLIS D E. Microstructure of Cu-C interface in Cu-based metal matrix composite[J]. Sensors and Actuators, 1999, 74: 86-90.
[4] MARQUES M T, CORREIA J B, CONDE O. Carbon solubility in nanostructured coppe r[J]. Scripta Materialia, 2004, 50: 963-967.
[5] L?PEZ G A, MITTEMEIJER E J. The solubility of C in solid Cu [J]. Scripta Materialia, 2004, 51: 1-5.
[6] SURYANARAYANA C. Mechanical alloying and milling [J]. Progress in Materials Science, 2001, 46: 1184.
[7] ZHANG D L. Processing of advanced materials using high-energy mechanical milling[J]. Progress in Materials Science, 2004, 49: 537-560.
[8] YAMANE T, OKUBO H, OKI N. Consolidation of mechanical alloyed powder mixture of Cu-Zn alloy and graphite[J]. Materials Science and Engineering A, 2003, 350: 173-178.
[9] BENJAMIN J S. Fundamentals of mechanical alloying[J]. Mater Sci Forum, 1992, 88-90: 1.
[10] BHATTACHARYA A K, ARZT E. Temperature rise during mechanical alloying[J]. Sci Metall Mater, 1992, 27: 749.
[11] LU L, SHEN Y F, CHEN X H, et al. Ultrahigh strength and high electrical conductivity in copper[J]. Science, 2004, 304: 422-426.
[12] LI Dou-xing, PING De-hai, HUANG Jian-yu. Microstructure in nanocrystalline materials[J]. Journal of Chinese Electron Microscopy Society, 1997, 16: 255-260. (in Chinese)
(Edited by CHEN Ai-hua)
Foundation item: Project (20030183067) supported by the Doctor Foundation of Ministry of Education of China; Project supported by the Education Depart-
ment Research Foundation of Jilin Province, China
Corresponding author: LIU Yong-bing; Tel: +86-13843022185; E-mail: wdxb1022@163.com