
Kinetics and mechanism of Re(Ⅶ) extraction and separation from Mo(Ⅵ) with trialkyl amine
LOU Zhen-ning(娄振宁), XIONG Ying(熊 英), SONG Jun-jun(宋俊俊), SHAN Wei-jun(单炜军),
HAN Guang-xi(韩光喜), XING Zhi-qiang(邢志强), KONG Yu-xia(孔玉霞)
College of Chemistry, Liaoning University, Shenyang 110036, China
Received 6 July 2009; accepted 30 December 2009
____________________________________________________________________________________________
Abstract: The extraction kinetics of rhenium(Ⅶ) or molybdenum(Ⅵ) with trialkyl amine (N235, R3N, R=C8–C10) dissolved in heptane were investigated by constant interfacial cell with laminar flow, which aimed to identify the extraction regime, reaction zone and rate equations. The influence of stirring speed, temperature, specific interfacial area, extraction concentration and chlorine concentration on the extraction of both metals was studied. It is concluded that the extractions of Re(Ⅶ) and Mo(Ⅵ) both take place at the liquid-liquid interface, while the extraction regimes are chemically-controlled for rhenium and mixed controlled for molybdenum, respectively. The extraction rate equations and the rate-determining step were obtained under the experimental conditions, and the extraction rate constant of Re(Ⅶ) or Mo(Ⅵ) with N235 was calculated. These obtained kinetics parameters are different between Re(Ⅶ) and Mo(Ⅵ), which provides better possibilities for Re(Ⅶ) and Mo(Ⅵ) separations at proper conditions.
Key words: extraction; kinetics; separation; N235; rhenium; molybdenum
____________________________________________________________________________________________
1 Introduction
Rhenium is usually recovered from molybdenite concentrates through roasting or direct reduction of the concentrates[1]. With the ever increasing demands of high purity rhenium and its compounds in petrochemical industry, national defense and aviation, high temperature emitter and space reactor, lots of methods are used to purify and separate these elements, such as chemical deposition, ion exchange, capillary electrophoresis, liquid chromatography and solvent extraction[2-6], of which liquid-liquid extraction provides an effective and simple separation method.
It is well known that extraction kinetics separation is a possibility for the quantitative separation of metal ions, which cannot be separated in the equilibrium state [7]. Although the thermodynamics of extraction are relatively well known, there is lack of thorough information on the kinetics of mass transfer in biphasic extraction systems[8-11]. Thus, the kinetics studies of the extraction of individual elements are definitely necessary for the development of the experimental procedure and accumulation of data, and necessary to understand the mechanism and mass-transfer models for a variety of extraction systems. Moreover, the revised Lewis cell, called the constant interfacial-area cell with laminar flow, developed by ZHENG et al[12] was used in our previous work[13]. The operation was carried out under laminar flow, which keeps the interface stable as a result of no flow between two phases.
In the present work, the extraction kinetics of Re(Ⅶ) with N235 dissolved in heptane using a constant interfacial cell with laminar flow is studied. The extraction controlling regime is carefully evaluated, and the reaction zone is determined by considering various effects on the extraction process. The purpose is to provide useful information towards developing more efficient and economical hydrometallurgy process for rhenium separation and purification from molybdenum.
2 Experimental
2.1 Reagent
Trialkyl amine (N235, R3N, R=C8-C10) was kindly supplied by Shanghai Organic Chemical Factory, China, and was used without purification. Rhenium stock solutions were prepared by dissolving NH4ReO4 (99.9%) in hydrochloric acid, and molybdenum stock solutions were prepared by dissolving (NH4)6Mo7O24·4H2O in hydrochloric acid. All other reagents were of analytical grade.
2.2 Procedure
The concentration of N235 in the organic phase and pH value of aqueous phase used in all experiments were 2.5×10-2 mol/L and 2.0, respectively, and varied when studying their effects on the rate. The aqueous phases contained 5.0×10-4 mol/L rhenium ion and 1.0×10-2 mol/L molybdenum ion, respectively. The interfacial area was 19.4 cm2. Both the volume of aqueous and organic phase was 98 mL. The extraction kinetics was investigated by using a constant interfacial area cell with laminar described previously[12]. The interfacial tension experiments were carried out by using JYZ-200 auto-tensiometer.
2.3 Theory
Assuming that the mass-transfer process can be formally treated as a pseudo-order reversible reaction with respect to the metal cation[14], one can write the following equation:
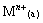

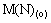 (1)
(1)
The following equations can be obtained:
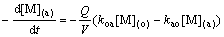
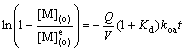 (3)
(3)
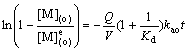 (4)
(4)
where kao is the forward pseudo-first-order rate constant; koa is the reverse pseudo-first-order constant; kd is the distribution constants of metal ion; Q is the interfacial area, m2; V is the volume of the aqueous phase or the organic phase, mL; (a) means aqueous phase; (o) means organic phase; and (e) means equilibrium of extraction.
The slopes of the plots of ln(1-[M](o)/[M]e(o)) vs t were used to evaluate koa and kao. All plots were straight lines in the work, indicating that the above assumption was reasonable.
3 Results and discussion
3.1 Stoichiometry of heterogeneous complex formation and decomposition reactions for Re(Ⅶ) and Mo(Ⅵ)
The stoichiometry of the complex formation reaction between the metal ion and N235 in heptane was evaluated by studying the dependence of lg D on the logarithmic N235 concentration at constant concentration of chloride ion, and lg D on the logarithmic concentration of chloride ion at constant extractant concentration, respectively. As shown in Figs.1 and 2, the slopes of lg D vs lg [N235] and lg D vs lg [Cl-] for rhenium ion are approximately equal to 1 and -1, respectively, and for molybdenum are also 1 and -1, respectively. Thus, the equilibrium equation of extraction reaction for Re(Ⅶ) and Mo(Ⅵ) using N235 can be proposed as:
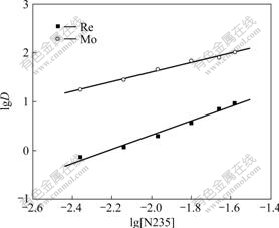
Fig.1 Dependence of distribution coefficient on extractant concentration(T=298 K, [Cl-]=0.1 mol/L, pH=2, [Re(Ⅶ)]=5×10-4 mol/L, [Mo(Ⅵ)]=1.0×10-3 mol/L)
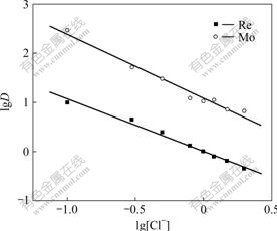
Fig.2 Dependence of distribution coefficient on chloride concentration(T=298 K, [N235]=2.5×10-2 mol/L, pH=2, [Re(Ⅶ)]=5×10-4 mol/L, [Mo(Ⅵ)]=1.0×10-3 mol/L)
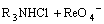 =
=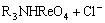 (5)
(5)
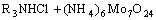 =
=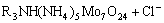 (6)
(6)
where R3NHReO4 and R3NH(NH)5Mo7O24 are organic complexes of two metal cations.
3.2 Extraction regime
In the extraction kinetics experiments, the criterion generally used to identify the extraction regime is independent of the extraction rate on the stirring speed in constant interfacial area cell. The effect of the stirring speed of the two phases on kao was studied at constant conditions for rhenium and molybdenum, respectively. The results obtained are shown in Fig.3. The initial linear dependence is found when the stirring speed is less than 250 r/min. The reason is due to the fact that at low stirring speed the thickness of the stagnant interfacial films is so large that the process of diffusion, which precedes the arrival of the metal species to the interface, is the slowest one. When a slow chemical reaction starts to become competitive with the diffusional process, the progressive increase of the stirring speed (higher than 250 r/min) which reduces the thickness of the stationary diffusional films will no longer cause proportional increase in extraction rate. Therefore, the presence of a “plateau region” in the extraction rate vs stirring speed curve is an indication that in that zone the extraction rate may be kinetics controlled.
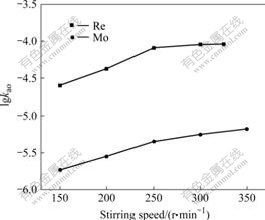
Fig.3 Effect of stirring speed on extraction rate(Q=19.4 cm2, V=98 mL, T=298 K, [N235]=2.5×10-2 mol/L, [Cl-]=0.1 mol/L, pH=2, [Re(Ⅶ)]=5×10-4 mol/L, [Mo(Ⅵ)]=1.0×10-3 mol/L)
Nevertheless, a “plateau region” can be also generated by other phenomena, and it is still possible that in spite of the experimentally determined independence of extraction rate on the stirring speed, the rate of extraction is still diffusion controlled or, at least not fully kinetics controlled. So, it is necessary to employ other approach as to identify the extraction regime.
A further criterion that enables distinguishing between a diffusion-controlled and a kinetics regime is the experimental determination of the activation energy of the extraction process. The effect of the temperature on the rhenium or molybdenum extraction rate was studied in the temperature range of 289-313 K. The apparent activation energy (Ea) for the extraction was calculated from the slope of lg kao vs T -1, as shown in Fig.4. The Ea was calculated as 27.47 and 44.23 kJ/mol for molybdenum and rhenium, respectively.
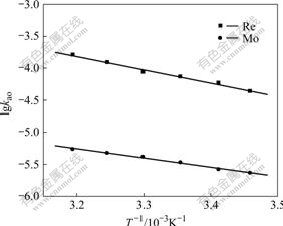
Fig.4 Effect of temperature on extraction rate(Q=19.4 cm2, V=98 mL, [N235]=2.5×10-2 mol/L, [Cl-]=0.1 mol/L, pH=2, [Re(Ⅶ)]=5×10-4 mol/L, [Mo(Ⅵ)]=1.0×10-2 mol/L, stirring speed 250 r/min)
In general, if the rate is controlled by a chemical reaction, Ea is more than 40 kJ/mol; if the rate is controlled by a diffusion process, Ea is less than 20 kJ/mol, and Ea value between 40 and 20 kJ/mol is expected for a mixed controlled regime. The obtained value of Ea of the extraction suggests a possible chemical reaction control regime both for molybdenum and rhenium extraction with N235 in the temperature range of 289-303 K.
All other extraction kinetics experiments were conducted at 250 r/min and 298 K in order to maintain the same hydrodynamic conditions.
3.3 Reaction zone
An important criterion to determine whether the chemical reactions that control the rate of extraction in a kinetic regime occur in the bulk phase or at the interface is the relationship between the extraction rate and the interfacial area. If the slow chemical reaction occurs in the bulk phases, the initial rate will be independent of interfacial area. On the contrary, a reaction occurring at the interface will show a direct proportionality between the rate and the interfacial area. The effect of the specific areas Q/V (interfacial area/phase volume) on the extraction rate (kao) was studied, and a linear relationship (shown in Fig.5) was obtained, which is the characteristic of an interfacial reaction for Re(Ⅶ) and Mo(Ⅵ) extraction with N235.
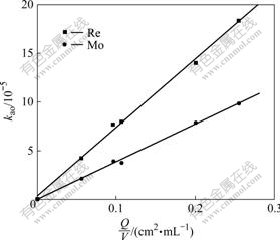
Fig.5 Effect of interfacial area on extraction rate(V=98 mL, T=298 K, [N235]=2.5×10-2 mol/L, [Cl-]=0.1 mol/L, pH=2, [Re(Ⅶ)]=5×10-4 mol/L, [Mo(Ⅵ)]=1.0×10-2 mol/L, stirring speed 250 r/min)
The chemical reaction occurring in interfacial zone is also supported by the studies of interfacial tension of extractant. Most solvent extraction reactions are interfacially absorbed and produce a lowering of the aqueous-organic diligent interfacial tension. Fig.6 shows the interfacial tension and interfacial excess vs extractant concentration plots on the basis of Gibbs and Szyszkowsi isotherm equations[15]. The equations are as follows:
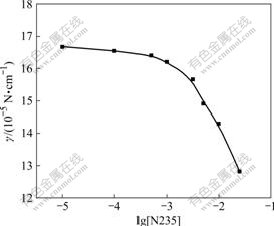
Fig.6 Effect of extractant concentration on interfacial tension (pH=2, [Cl-]=0.1 mol/L, T=298 K)
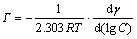 (7)
(7)
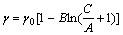 (8)
(8)
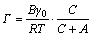 (9)
(9)
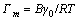 (10)
(10)
where Г is the interfacial excess, mol/cm2; C is the extractant concentration, mol/L; γ is the interfacial tension, 10-5 N/cm.
The values of Γm (maximum interfacial excess), Ai (adsorptive molecular area in interface) and Cmin (minimal concentration at saturation) are obtained as follows: Γm=7.67×10-7 mol/cm, Ai=2.16 nm2, Cmin= 5.0×10-4 mol/L, which show high interfacial activity of extractant. Therefore, the strong surface activity of N235 at hepane-water interfaces makes the liquid-liquid interface the most probable local for the chemical reactions.
3.4 Extraction rate equation for Re(Ⅶ) and Mo(Ⅵ)
The effects of the concentration of chloride ion in the aqueous phase and the concentration of extractant on extraction rate are shown in Figs.7 and 8, respectively. A linear correlation between lg kao and lg[N235] indicates that the order of extractant for Re(Ⅶ) extraction is 0.91, and 0.71 for Mo(Ⅵ). A linear correlation between lg kao and lg[Cl-] indicates that the order of chloride ion for Re (Ⅶ) extraction is -0.71, and -0.74 for Mo(Ⅵ).
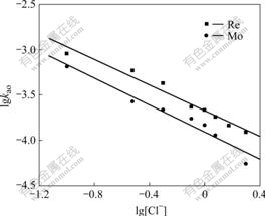
Fig.7 Effect of chloride concentration on extraction rate (Q=19.4 cm2, V=98 mL, T=298 K, [N235]=2.5×10-2 mol/L, pH = 2, [Re(Ⅶ)]=5×10-4 mol/L, [Mo(Ⅵ)]=1.0×10-2 mol/L, stirring speed 250 r/min)
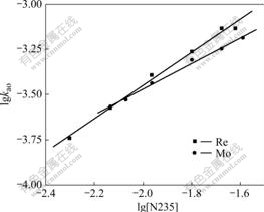
Fig.8 Effect of extractant concentration on extraction rate(Q= 19.4 cm2, V=98 mL, T=298 K, [Cl-]=0.1 mol/L, pH=2, [Re(Ⅶ)]=5×10-4 mol/L, [Mo(Ⅵ)]=1.0×10-2 mol/L, stirring speed 250 r/min)
According to the above results, the rate equations for Re(Ⅶ) and Mo(Ⅵ), respectively, of extraction with N235 at the experimental condition can be written as:
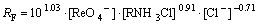 (11)
(11)
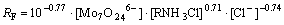 (12)
(12)
The chemical reaction for the extraction of Re(Ⅶ) with N235 at the liquid-liquid interface is mentioned above. Referring to the interfacial reaction model proposed by DANESI et al[16], the following reactions will be considered:
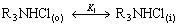 (13)
(13)
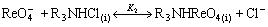 (14)
(14)
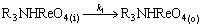 (15)
(15)
Considering Eq.(15) as the rate-controlling steps, one can write the following equations:
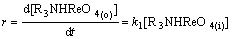 (16)
(16)
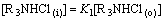 (17)
(17)
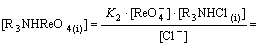
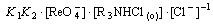 (18)
(18)
Based on Eqs.(16), (17) and (18), one can write the initial rate of extraction:
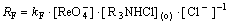 (19)
(19)
where kF = k3·K1·K2
The above mechanism is consistent with the rate Eq.(11) obtained from experimental results. The predictions derived from interfacial-reaction models have been found to be in good agreement with the rate equations obtained from experimental data, confirming the basic assumption that the chemical reaction is located at the liquid-liquid interface and the extraction rate is controlled by the step of reaction (15) interfacial chemical reactions.
3.5 Separation of Re(Ⅶ) or Mo(Ⅵ) with N235
The extraction kinetics of Mo(Ⅵ) with N235 was studied under the same experimental conditions of Re(Ⅶ) extraction. The values of extraction rate constant for the extraction of Mo(Ⅵ) or Re(Ⅶ) are summarized in Table 1, and compared with the thermodynamic extraction result from the values of separation and factor (β). The data indicate that the separation of Mo(Ⅵ) and Re(Ⅶ) at a proper extraction time, pH and concentration of extractant can be easier to complete by kinetics method than by equilibrium extraction, confirmed by the larger value of ?kF (defined as ?kF=kF(Re)/kF(Mo)=63.3) than the value of β(DMo/DRe=14.08).
Table 1 Extraction rate constant (kF) and separation factor (β) for extraction of Mo(Ⅵ) and Re (Ⅶ)

4 Conclusions
1) The extraction of Re(Ⅶ) with N235 in heptane using a constant interfacial area cell with laminar flow is a chemically controlled kinetics process with an interfacial reaction. The similar result is obtained in the case of Mo(Ⅵ) extraction.
2) The data were analyzed in terms of pseudo-first order constant. The dependence of extraction rate on species concentration was studied and the rate equations were deduced.
3) By comparing the results of kinetics (?kF) with that of thermodynamic (β), it provides better possibilities and separation effect for Re (Ⅶ) and Mo(Ⅵ) at a kinetic conditions.
References
[1] SUTULOV A. Molybdenum and rhenium recovery from porphyry coppers[M]. University of Concepcion, Chile, 1970: 1.
[2] ASKARI ZAMANI M A, HIROYOSHI N, TSUNEKAWA M, VAGHAR R, OLIAZADEH M. Bioleaching of Sarcheshmeh molybdenite concentrate for extraction of rhenium[J]. Hydrometallurgy, 2005, 80: 23-31.
[3] MOZAMMEL M, SADRNEZHAAD S K, BADAMI E, AHMADI E. Breakthrough curves for adsorption and elution of rhenium in a column ion exchange system[J]. Hydrometallurgy, 2005, 85: 17-23.
[4] LAN X, LIANG S, SONG Y. Recovery of rhenium from molybdenite calcine by a resin-in-pulp process[J]. Hydrometallurgy, 2006, 82: 133-136.
[5] YAMINI Y, SALEH A, KHAJEH M. Orthogonal array design for the optimization of supercritical carbon dioxide extraction of platinum(IV) and rhenium(VII) from a solid matrix using cyanex 301[J]. Separation and Purification Technology, 2008, 61: 109-114.
[6] CAO Z F, ZHONG H, QIU Z H. Solvent extraction of rhenium from molybdenum in alkaline solution[J]. Hydrometallurgy, 2009, 97: 153-157.
[7] ITABASHI H, TAKAZAWA Y, NIIBE N, KAWAMOTO H. Kinetics separation of cadmium(II) from Zinc(II) with dithizone by back-extraction[J]. Anal Sci, 1997, 13: 921-928.
[8] CHITRA K R, GAIKWAD A G, SURENDER G D, DAMODARAN A D. Studies on kinetics of forward and backward extraction of neodymium by using phoshonic acid monoester as an acidic extraction[J]. The Chemical Engineering Journal, 1995, 60: 63-67.
[9] WU Dong-bei, XIONG Ying, LI De-qian, MENG Shu-lan. Interfacial behavior of Cyanex 302 and kinetics of lanthanum extraction[J]. Journal of Colloid and Interface Science, 2005, 290(1): 235-240.
[10] BISWAS R K, HABIB M A, MONDAL M G K. Kinetics and mechanism of stripping of Mn(II)–D2EHPA complex by sulphuric acid solution[J]. Hydrometallurgy, 2005, 80(3): 186-195.
[11] CORSI C, GNAGNATELLI G, SLATER M J, VEGLIO F. A study of the kinetics of zinc stripping for the system Zn/H2SO4/D2EHPA/ n-heptane in a hancil constant interface cell and a rotating disc contactor[J]. Hydrometallurgy, 1998, 50: 125-141.
[12] ZHENG Z, LU J, LI D Q, MA G X. The kinetics study in liquid-liquid systems with constant interfacial area cell with laminar flow[J]. Chem Eng Sci, 1998, 53(13): 2327-2333.
[13] XIONG Y, WANG Y G, LI D Q. Kinetics of extraction and stripping of Y(III) by Cyanex 272 as an acidic extractant using a constant interfacial cell with laminar flow[J]. Solvent Extr Ion Exch, 2004, 22(5): 833-851.
[14] DANESI P R, VANDERGRIFT G F. Kinetics and mechanism of the interfacial mass transfer of Eu3+ and Am3+ in the system bis(2-ethylhexyl) phosphate-n-dodecane-NaCl-HCl-water[J]. J Phys Chem, 1981, 85: 3646-3651.
[15] SHEN Jing-lan, XI Zheng-kai, GAO Zi-li, SUN Si-xiu. Studies on the interfacial properties of liquid–liquid extraction. II. Interfacial properties of HEH (EHP) in water-diluent system[J]. Chin J Appl Chem, 1984, 1(4): 57-62. (in Chinese)
[16] DANESI P R, CHIARIZIA R. The kinetics of metal solvent extraction[J]. CRC Crit Rev Anal Chem, 1980, 10: 1-126.
Foundation item: Project(20701017) supported by the National Natural Science Foundation of China
Corresponding author: XIONG Ying; Tel: +86-24-62202006; Fax: +86-24-62202006; E-mail: xiongying_1977@hotmail.com
(Edited by YUAN Sai-qian)