
HIP diffusion bonding of P/M titanium alloy Ti-6Al-4V
and stainless steel 1Cr18Ni9Ti
LANG Ze-bao(郎泽保), WANG Liang(王亮), ZHANG Xu-hu(张绪虎)
Aerospace Research Institute of Materials and Processing Technology, Beijing 100076, China
Received 15 July 2007; accepted 10 September 2007
Abstract: The HIP diffusion bonding of P/M titanium alloy Ti-6Al-4V and stainless steel 1Cr18Ni9Ti using pure Ni as intermediate layer was studied. Bonding joint with complex bonding interface was obtained by HIPing pre-alloyed Ti-6Al-4V powders and stainless steel 1Cr18Ni9Ti in a vacuum canning. The joint strengths were examined and the characteristics of bonding joint were observed. The result shows that the maximized strength of HIP diffusion bonding between P/M titanium alloy Ti-6Al-4V and stainless steel 1Cr18Ni9Ti can be up to 388 MPa and the microstructure of bonding joint is acceptable.
Key words: Ti-6Al-4V; 1Cr18Ni9Ti; HIP diffusion bonding; stainless steel; titanium alloy; powder metallurgy
1 Introduction
The bonding joint between titanium alloy and stainless steel has become one of the most attractive materials in the aerospace due to the combination of the characteristics of titanium alloy and stainless steel[1]. However, in the operations of fusion bonding, intermetallics, i.e., TiFe and TiFe2, can appear in bonding interface, which result in poor joint strength. In additional, the difference of linear expansibility between titanium alloy and stainless steel can also lead to internal stress in bonding joint and even failure of the joint [2-3].
The general method to deal with this problem is using intermediate layer between titanium alloy and stainless steel to avoid the formation of brittle intermetallics. Typical intermediate layer for diffusion bonding of titanium alloy and stainless steel are Cu, V and Ni with Ni being the most common intermediate layer own to its high property of corrosion resistant, infinite solution with Fe and forming ductile intermetallics with element Ti [4-5]. Thus, Ni was also selected as intermediate layer in this study.
Typical diffusion bonding technologies include such technologies as diffusion bonding at constant temperature and pressure, phase transformation super plastic diffusion bonding, diffusion bonding under pulsatile pressure and HIP (hot isostatic pressing) diffusion bonding [6]. These methods have a highly assembling requirement of interfaces, which makes diffusion bonding of different metals with complex interfaces difficult. However, spherical powders can meet the assembling requirement of diffusion bonding interfaces for their favorable fluidity. In the following HIP processing, powders can be condensed and diffusion bonded with other metals. Thus, diffusion bonding with complex interface can be obtained by HIPing spherical powders and different metals can be loaded in a vacuum can for combining the consolidation of powders and element diffusion. The present paper is based partly on the study of diffusion bonding of titanium alloy and stainless steel with complex interface.
2 Experimental
The stainless steel 1Cr18Ni9Ti and pre-alloyed Ti-6Al-4V powders were selected for present study, whose chemical compositions are listed in Table 1. Pure Ni was used as intermediate layer for diffusion bonding in this investigation. The experiment was performed in QIH-32 HIP equipment made in Sweden.
Table 1 Chemical composition of based materials (mass fraction, %)
In the experiment, stainless steel 1Cr18Ni9Ti was machined to cylinder and electroplated pure Ni with different thickness on its surface. For HIP diffusion bonding, the stainless steel 1Cr18Ni9Ti cylinder and pre-alloyed Ti-6Al-4V powers were loaded in a mild steel can with tapping, evacuated at 573-773 K and subsequently sealed. The HIP diffusion bonding of P/M titanium alloy Ti-6Al-4V and stainless steel 1Cr18Ni9Ti in this study was performed at 1 073-1 173 K and 100 MPa for 2 h.
3 Results and discussion
3.1 Effects of HIP temperatures and intermediate layer thickness on joint strength
The joint strength of HIP diffusion bonding depends on the microstructures of the joint and some other factors, such as HIP temperatures, pressures, HIP times and thickness of intermediate layer, have a great effect on the microstructures [7-8]. In the experiment, different HIP temperatures and different intermediate layer thickness were involved to pursue a novel approach for HIP diffusion bonding of P/M titanium alloy Ti-6Al-4V and stainless steel 1Cr18Ni9Ti. The bonding joint was obtained (Fig.1) and the joint strength was tested and the results are listed in Table 2. From the data of Table 2, the joint strength of HIP diffusion bonding of P/M titanium alloy Ti-6Al-4V and 1Cr18Ni9Ti reaches 388 MPa, and the novel method for HIP diffusion bonding of P/M titanium alloy Ti-6Al-4V and 1Cr18Ni9Ti is performing the bonding at the temperature of 1 073-1 093 K and with the intermediate thickness of 40 μm. After the HIP processing, the joint obtains a higher tensile strength than that after common diffusion bonding[9].
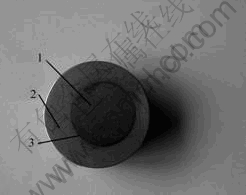
Fig.1 Photograph of bonding joint between P/M titanium alloy Ti-6Al-4V and stainless steel 1Cr18Ni9Ti: 1—1Cr18Ni9Ti stainless steel; 2—Ti-6Al-4V alloy; 3—Bonding interface
Table 2 Influence of HIP temperatures and intermediate layer thickness on HIP diffusion joint strength
3.2 Characteristics of tensile fracture
The tensile fracture of samples was investigated. It is found that all samples have broken in the area of diffusion joint and have no significant sign of necking. Fig.2 shows the photographs of tensile fracture at different HIP temperatures and with different thickness of intermediate layer. The spherical blocks locate on the surface of tensile fracture uniformly and some chips with grey color appear in the edge areas. SEM was applied to characterize the tensile fractures of samples, as shown in Fig.2. The results are shown in Fig.3. Blocks of the fracture are formed by many fine and homogeneous grains and the background region looks like river patterns. These characters show that the fracture of block region occurs at the edge of the grain and the fracture of background region is a traditional cleavage fracture [10-11].
3.3 Microstructures of bonding joint
The joint strength of HIP diffusion bonding between P/M titanium alloy Ti-6Al-4V and 1Cr18Ni9Ti depends on the microstructure of the joint. Thus, the microstructures of joint metallographic were emphasized in the investigation. The metallographic photo for bonding joint after corrosion is shown in Fig.4. According to Fig.4, the joint of titanium alloy Ti-6Al-4V
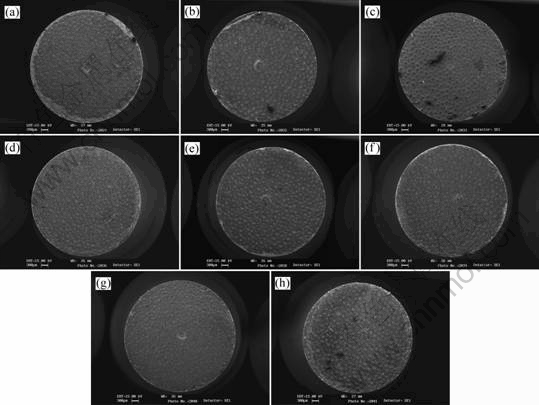
Fig.2 Micrographs of tensile fractures at different HIP temperatures and with different thickness of intermediate layer: (a) T=1 073- 1 09 3K, δ=40 μm; (b) T=1 073-1 093 K, δ=80 μm; (c) T=1 073-1 093 K, δ=120 μm; (d) T= 1 123-1 143 K, δ=40 μm; (e) T= 1 123-1 143 K, δ=80 μm; (f) T=1 123-1 143 K, δ=120 μm; (g) T=1 123-1 143 K, δ=80 μm; (h) T=1 123-1 143 K, δ=120 μm (T is used for HIP temperature and δ is used for thickness of intermediate layer)
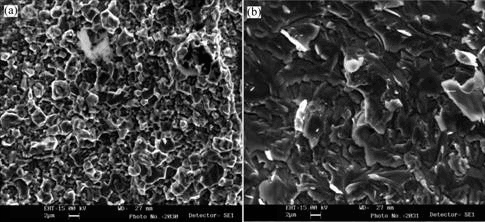
Fig.3 SEM images of different regions of fracture: (a) Block region; (b) Background region
and stainless steel 1Cr18Ni9Ti can be divided into five regions: region 1 is the basis region of titanium alloy Ti-6Al-4V; region 2 is a narrow region of transition; region 3 is the solid solution of Fe, Cr, Ni and Ti; region 4 is the region of electric plating Ni and region 5 is the basis region of stainless steel 1Cr18Ni9Ti. Each region is bonded well and no flaw is found. The compositions of Ti, Fe, Cr and Ni of these five regions were tested by electronic probe and the results are shown in Fig.5.
As shown in Fig.5, the element Ni diffuses significantly towards the region of titanium alloy Ti-6Al-4V and the composition of element Ti deceases from region 1 to region 5 and has a suddenly reduction at interface of 1Cr18Ni9Ti/Ni due to the low solution of element Ti in region 1Cr18Ni9Ti [12]. The element Fe enriches in the solid solution region and element Cr has a low diffusion during HIP processing. The reason for the distributions of these elements is that different elements have different activation energies and different sizes of atomic radius, which result in different diffusion velocities and different diffusion quantities. The diffusion quantity of these elements in the joint region results in the phase composition of joint and has a great effect on the joint strength. From above discussion, the conclusion can be obtained that it is very important to control the quantity and distribution of these elements [13].
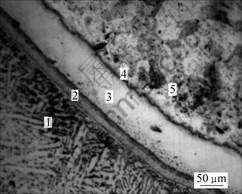
Fig.4 Joint metallograph of Ti-6Al-4V/1Cr18Ni9Ti
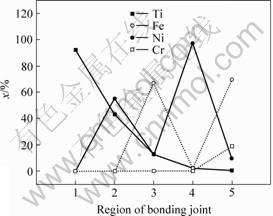
Fig.5 Distributions of Ti, Fe, Cr and Ni in diffusion bonding interface: Regions 1-5 shown in Fig.4
3.4 Properties of P/M Ti-6Al-4V appeared in HIP diffusion bonding
The P/M Ti-6Al-4V alloy was obtained by HIPing pre-alloyed Ti-6Al-4V powders during HIP diffusion bonding. The mechanical properties of P/M Ti-6Al-4V were examined, as listed in Table 3. The P/M Ti-6Al-4V has a high tensile strength that surpasses the standards in GB/T 2965—1996 of China. The microstructures are shown in Fig.6. The P/M Ti-6Al-4V performs a finer, homogenous microstructure than forging or casting Ti-6Al-4V alloys [14-16].
Table 3 Mechanical properties of P/M Ti-6Al-4V

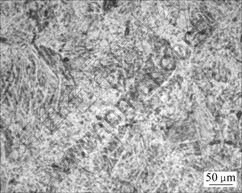
Fig.6 Metallographic photo of P/M Ti-6Al-4V
4 Conclusions
1) The bonding joint with complex bonding interface can be obtained by HIP diffusion bonding of P/M titanium alloy Ti-6Al-4V and stainless steel 1Cr18Ni9Ti.
2) The highest joint strength reaches 388 MPa when the specimen is bonded at 1 073-1 093 K and with the thickness of intermediate layer of 40 μm.
3) The joint obtained in this study is bonded well and no flaw is found. Thus, HIP diffusion bonding of P/M titanium alloy Ti-6Al-4V and stainless steel can be applied to process the bonding joint with complex interfaces.
References
[1] GHOST M, CHATTERJEE S, MISHRA B. The effect of intermatallics on the strength properties of diffusion bonds formed between Ti-5.5Al-2.4V and 304 stain less steel[J]. Mater Sci Eng A, 2003, A363: 268-274.
[2] SONG Min-xia, ZHAO Xi-hua, GUO Wei, FENG Ji-cai. Developments and present situation of diffusion bonding of titanium alloy to other metals[J]. Welding & Joining, 2005(1): 5-7.
[3] Kale G B, Patil R V, Gawade P S. Interdiffusion studies in titanium-304 stainless steel system[J]. Journal of Nuclear Materials, 1998, 257(1): 44-50.
[4] SUN Rong-lu, LI Mu-qin, ZHANG Jiu-hai, HUANG Xi-Dong. Influence of different transition metals on properties of diffusion bonding joint of Ti alloy to stainless steel[J]. Transactions of the China Welding Institution, 1996, 17(4): 212-218.
[5] Kundu S, Ghosh M, Laik A, Bhanumurthy K, Kale G B, Chatterjee S. Diffusion bonding of commercially pure titanium to 304 stainless steel using copper interlayer [J]. Mater Sci Eng A, 2005, 407(1/2): 154-160.
[6] QIN Bin, SHENG Guang-min, ZHOU Bo, HUANG Jia-wei, LI Cong, QIU Shao-yu. Diffusion welding of titanium alloy and stainless steel[J]. The Chinese Journal of Nonferrous Metals, 2004, 14(9): 1545-1550. (in Chinese)
[7] HE Feng, WANG Wu-xiang, LI Cheng-gong. Study of P/M superalloy blisk prepared by HIP diffusion bonding[C]//Anoshkin N, Garibov G, Fatkullin O, Kachanov Y E, Kazberovich A, Samarov V, Yermanko M, ed. Proceedings of international conference on HIP 02. Moscow: All Russia Institute of Light Alloy, 2002: 55-59.
[8] ZHANG Ying-cai, CHEN Hong. Study on HIP diffusion bonding of titanium/steel composite[J]. Rare Metals, 1994, 18(6): 463-466.
[9] Ghosh M, Chatterjee S. Diffusion bonded transition joints of titanium to stainless steel with improved properties[J]. Mater Sci Eng A, 2003, 358: 152-158.
[10] QIN Bin, SHENG Guang-min, HUANG Jia-wei ZHOU Bo, QIU Shao-yu, LI Cong. Fracture analysis of diffusion–bonded joint between titanium alloy and stainless steel [J]. Iron Steel Vanadium Titanium. 2005, 26(4): 55-59
[11] GHOSH M, CHATTERJEE S. Characterization of transition joints of commercially pure titanium to 304 stainless steel[J]. Materials Characterization, 2002, 48(5): 393-399.
[12] SUN Rong-lu, YANG Wen-jie, ZHANG Jiu-hai. The microstructure and properties of titanium alloy and stainless steel diffusion bonding[J]. Welding Technology, 1997, 4: 4-6.
[13] SUN Rong-lu, ZHANG Jiu-hai, HUANG Xi-dong. Element diffusion in the joint of Ti-Ni-stainless steel and its influence on the properties of diffusion bonding joint[J]. Mater Sci Technol, 1996, 4(4): 63-67.
[14] WANG Liang, SHI Hong-pei. Research of high performance titanium alloy powder metallurgy technology[J]. Aerospace Materials & Technology, 2003, 3: 42-44.
[15] Froes F H, Eylon D. Titanium powder metallurgy–A review[C]//Froes F H, Eylon D, ed. Titanium Net Shape Technologies. Pennsylvania: The Metallurgical Society of AIME, 1984: 1-20.
[16] Sheinker A A, Chananic G R, Bohlen J W. Evaluation and application of prealloyed titanium P/M parts for airframe structures[J]. Int J Powder Metall, 1987, 23(3): 171-179.
Foundation item: Projects (51312010310) supported by the General Armament Department of Chinese PLA
Corresponding author: LANG Ze-bao; Tel: +86-010-68380666; E-mail: lzb0413@sina.com.cn
(Edited by HE Xue-feng)