蒽醌-2-磺酸钠介导下奥奈达希瓦氏菌还原U(VI)
来源期刊:中国有色金属学报(英文版)2015年第12期
论文作者:刘金香 谢水波 王永华 刘迎九 蔡萍莉 熊芬 王文涛
文章页码:4144 - 4150
关键词:U(VI);蒽醌-2-磺酸钠(AQS);奥奈达希瓦氏菌;XPS
Key words:U(VI); anthraquinone-2-sulfonate (AQS); Shewanella oneidensis; XPS
摘 要:在厌氧环境下,以蒽醌-2-磺酸钠(AQS)作为腐殖质模式物,对奥奈达希瓦氏菌(Shewanella oneidensis,S. oneidensis)还原U(VI)的效果与主要影响因素进行研究。结果表明:在30 °C,当pH值为7.0,AQS为1.0 mmol/L,反应96 h后,S. oneidensis对U(VI)的去除率达到99.0%。AQS浓度为0.5~1.0 mmol/L时,其能有效促进U(VI)的还原,当AQS浓度超过2.0 mmol/L时,其对U(VI)的还原促进作用明显减小。Cu2+、Cr6+和Mn2+对U(VI)的还原均表现出明显的抑制作用,而Zn2+对U(VI) 的还原影响较小。NO3-离子浓度低于0.5 mmol/L时,其对U(VI)的还原基本没有影响,而当NO3-离子浓度高于1.0 mmol/L时,其对U(VI)的还原存在明显的抑制作用。当SO42-离子浓度低于5.0 mmol/L时,其对U(VI)的还原有微弱的促进作用。在AQS+S. oneidensis+ U(VI)还原体系中投加零价铁(ZVI),能快速促进U(VI)的还原,且U(VI)的还原率与ZVI的投加量(0~2.0 mg/L)呈正相关。XPS分析结果表明:菌体表面沉积U(VI)和U(IV)两种价态的U元素,反应后的铀主要形成稳定的UO2。
Abstract: Anthraquinone-2-sulfonate (AQS) was employed in humus substitutes to evaluate the effects and influencing factors of U(VI) reduction by Shewanella oneidensis MR-1 (S. oneidensis MR-1) under anaerobic condition. The removal rate of U(VI) at 30 °C reaches 99.0% afterd 96 h with the pH value of 7.0 and AQS concentration of 1.0 mmol/L. The effective concentrations of AQS as the accelerator for U(VI) bioreduction are approximately 0.5-1.0 mmol/L. The bioreduction of U(VI) is inhibited when the concentration of AQS exceeds 2.0 mmol/L. The coexistence of ions, such as Cu2+, Cr6+, Mn2+, shows a remarkable negative effect on the U(VI) reduction, and Zn2+ shows less influence on the process compared with other tested ions. The U(VI) reduction is remarkably inhibited when the concentration of nitrate ion exceeds 1.0 mmol/L. Otherwise, no difference is found when the nitrate ion concentration is less than 0.5 mmol/L.Sulfate ion (<5.0 mmol/L) slightly promotes the U(VI) reduction. Zero-valent iron (ZVI) promotes the U(VI) reduction by S. oneidensis, and the reduction rate improves with increasing the amount of ZVI in the range of 0-2.0 g/L. The XPS result indicates that uranium deposits on the cell surface are in U(VI) and U(IV) forms, and the majority of uranium in the solution is stableUO2.
Trans. Nonferrous Met. Soc. China 25(2015) 4144-4150
Jin-xiang LIU1, Shui-bo XIE 1,2, Yong-hua WANG1, Ying-jiu LIU1, Ping-li CAI1, Fen XIONG1, Wen-tao WANG1
1. Hunan Province Key Laboratory of Pollution Control and Resources Reuse Technology, University of South China, Hengyang 421001, China;
2. Key Discipline Laboratory for National Defence for Biotechnology in Uranium Mining and Hydrometallurgy, University of South China, Hengyang 421001, China
Received 2 February 2015; accepted 29 August 2015
Abstract: Anthraquinone-2-sulfonate (AQS) was employed in humus substitutes to evaluate the effects and influencing factors of U(VI) reduction by Shewanella oneidensis MR-1 (S. oneidensis MR-1) under anaerobic condition. The removal rate of U(VI) at 30 °C reaches 99.0% afterd 96 h with the pH value of 7.0 and AQS concentration of 1.0 mmol/L. The effective concentrations of AQS as the accelerator for U(VI) bioreduction are approximately 0.5-1.0 mmol/L. The bioreduction of U(VI) is inhibited when the concentration of AQS exceeds 2.0 mmol/L. The coexistence of ions, such as Cu2+, Cr6+, Mn2+, shows a remarkable negative effect on the U(VI) reduction, and Zn2+ shows less influence on the process compared with other tested ions. The U(VI) reduction is remarkably inhibited when the concentration of nitrate ion exceeds 1.0 mmol/L. Otherwise, no difference is found when the nitrate ion concentration is less than 0.5 mmol/L. Sulfate ion (<5.0 mmol/L) slightly promotes the U(VI) reduction. Zero-valent iron (ZVI) promotes the U(VI) reduction by S. oneidensis, and the reduction rate improves with increasing the amount of ZVI in the range of 0-2.0 g/L. The XPS result indicates that uranium deposits on the cell surface are in U(VI) and U(IV) forms, and the majority of uranium in the solution is stable UO2.
Key words: U(VI); anthraquinone-2-sulfonate (AQS); Shewanella oneidensis; XPS
1 Introduction
Uranium-contaminated groundwater is a key issue in uranium mining and metallurgy. This groundwater contains radioactive pollutants (uranium), heavy metal ions (Cu2+, Cr6+, Mn2+ and Zn2+), anions (NO3- and SO42-), and toxic organics [1,2]. The treatment of uranium-contaminated wastewater using conventional processes based on chemical and physical methods is limited by high cost, chemical wastes, and complex subsequent treatment. Alternative approaches, such as biosorption, bioacummulation, and biomineralization, have been considered for uranium remediation using microorganisms. Contrary to traditional processes, bioremediation methods are less expensive, having high removal efficiencies, and pollution-free, so this alternative process can be applied to treat uranium- contaminated wastewater [3].
Shewanella oneidensis (S. oneidensis) is a facultative anaerobic organism that can survive at low environmental temperature of 4 °C and in anaerobic environment of groundwater or deposition with rich organic matter in uranium mining regions. This organism has attracted much attention in the reductive bioremediation of uranium-contaminated wastewater because it could grow aerobically or anaerobically on a vast array of electron acceptors, such as U(VI), Pd, Pu, Mn(IV), Fe(III), Cr(VI), nitrates, and organic pollutants [4-8]. Recent studies have indicated that S. oneidensis reduced aqueous U(VI) to insoluble U(IV) with the precipitation of immobile uraninite (UO2) in contaminated groundwater. LOVELY et al [9] found the vast existence of microbial humus respiration in soil, sediment, or other anaerobic environments. They also showed that humic acid acting as a medium for electronic transformation can effectively promote Fe(III) reduction under experimental conditions with the coexistence of humic reductive bacteria and humic acid. Humus can also promote the reductive precipitation of U(VI), Cr(VI), and other heavy metals [10-12]. This organic matter can also enhance the biodegradation of azo dyes [13] and other toxic organic compounds.
Most studies have focused on the biodegradation of toxic organic compounds, such as azo dyes, using Shewanella [14-16]. However, the studies on reductive precipitation of aqueous U(VI) are highly important for biological remediation of uranium-contaminated wastewater. In this work, S. oneidensis was employed as the model organism, and its effects and major factors for U(VI) reduction in the presence of anthraquinone- 2-sulfonate (AQS) were examined.
2 Experimental
2.1 Organism and culture medium
S. oneidensis MR-1 was provided by Marine Culture Collection of China (No. 1A01706).
The basal media consisted of 2.5 g/L NaHCO3, 0.1 g/L KCl, 0.25 g/L NH4Cl, 0.1 g/L NaCl, 0.04 g/L KH2PO4, 0.05 g/L MgSO4·7H2O, 0.2 g/L MgCl2·6H2O, and 1.0 g/L yeast extract. A certain amount of sodium lactate was added into the above culture medium as the electron donor in the reduction experiments.
2.2 U(VI) reduction by S. oneidensis in presence of AQS
Figure 1 illustrates the test equipment. The experiments were conducted in 150 mL conical flasks, which contained 100 mL microbial medium that consisted of sterilized basal media, 10.0 mmol/L sodium lactate, 20.0 mg/L U(VI) and 1.0 mmol/L AQS. The pH value of the medium was subsequently adjusted to 7.0 by using NaOH or HCl. Pure nitrogen and carbon dioxide were bubbled through bacteria filter into the conical flasks of the culture media for 15 min to remove the oxygen inside the bottles. The bacterial suspension, of which the optical density value in the wavelength of 600 nm (OD600) was 0.81, with the volume ratio of 2% was inoculated into the above culture media under the protection of mixed gases. Finally, the bottle mouths were sealed with butyl rubber stoppers, and the bacterial suspensions were incubated at 30 °C on an orbital shaker at 120 r/min. All experiments were performed under the aforementioned experimental conditions. Additionally, subsequent controls were incubated along with the treatment groups. The samples with similar volumes were extracted with a syringe from the sampling tube at regular intervals, and the mixed gases were again flushed into bottles to maintain anaerobic conditions during the experiment. The supernatant samples obtained through 0.45 μm filtration membrane after centrifugation at 8000 r/min for 10 min were analyzed.
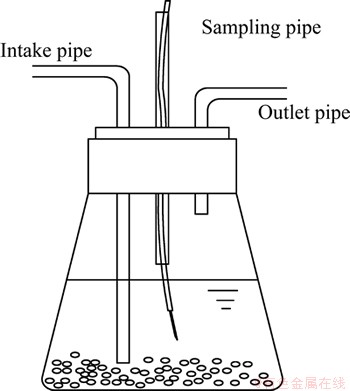
Fig. 1 Anaerobic culture device
The influencing factors of U(VI) reduction by S. oneidensis in the presence of AQS were investigated under the following conditions: pH values of 4.0, 5.0, 6.0, 7.0 and 8.0, coexistent metal ions of Cu2+, Zn2+, Cr6+ and Mn2+, main anions of NO3- (0.2, 0.5, 1.0 and 2.0 mmol/L) and SO42- (0.2, 1.0, 2.0 and 5.0 mmol/L), and zero-valent iron (ZVI) dosages of 0, 0.5, 1.0 and 2.0 g/L. Each experimental group set had three parallel experiments, and the average values from the tests were recorded as the experimental results. The same group of microorganism was used at each test to ensure experiment comparability.
2.3 Main reagents and analytical methods
The main reagents were uranosouranic oxide (U3O8, analytical grade), standard uranium solution prepared according to GB W04201, and AQS (analytic grade, Sigma Company). The iron powder was of analytical grade and obtained from Tianjin Chemical Reagent Factory, China. All other reagents were of analytical grade, and ultra-pure water was used in the experiment.
Spectrophotometric method according to national standard GB 6768-86 was used to determine trace uranium. The removal rate of U(VI) (RU(VI)) was obtained using the following equation:
RU(VI)=(A0-A1)/A0×100% (1)
where A0 and A1 are the concentrations of U(VI) in the solution before and after the reaction, respectively.
3 Results and discussion
3.1 Tolerance of S. oneidensis for U(VI)
The tolerance of the microorganism to uranium was investigated. The experiments were performed at U(VI) concentrations of 0, 20, 50, 80, 120 and 160 mg/L. The results are shown in Fig. 2. In Fig. 2, the bacteria grow and reproduce in the basal media without uranium. The organisms grow slowly in the early growth phase because of the lack of nutrients from the media. Then, after this short period of stagnation, the bacteria quickly grow into logarithmic period, and the OD600 values of the bacteria are 0.42 after 24 h and 0.97 after 36 h. The bacterial growth is significantly inhibited, and the bacteria need an extended period to adapt to the environment when the uranium concentration is 20 mg/L, in which OD600 is only 0.05 after 24 h and 0.73 after 60 h. When the uranium concentration increases to 50 mg/L, the uranium causes a toxic effect on the organisms, so that the organisms grow very slowly with OD600 of only 0.39 after 60 h. When U(VI) concentration is more than 80 mg/L, the bacterial structure is damaged, and the bacteria even die [17]. The effects of U(VI) concentration on S. oneidensis are distinct, and S. oneidensis tolerate toxicity at uranium concentrations below 50.0 mg/L.
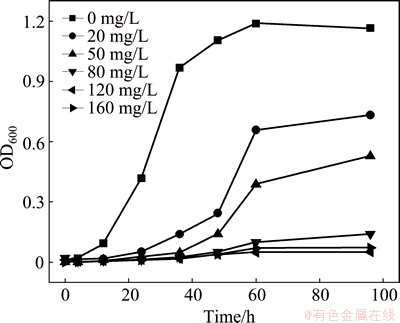
Fig. 2 Resistance of S. oneidensis on uranium concentration
3.2 U(VI) reduction in different redox systems
Control groups were prepared to accurately verify the contributions of AQS and microorganisms to U(VI) reduction. The control groups consisted of bottles with sole 1.0 mmol/L AQS or bacteria. The effects of different AQS concentrations on U(VI) reduction were evaluated. The experiments with both bacteria and AQS were performed with AQS concentrations of 0.5, 1.0 and 2.0 mmol/L. The results are shown in Fig. 3. AQS or bacteria each could reduce U(VI) in the solution. In the control group with only bacteria, approximately 40% of U(VI) is reduced after 4 d. In the group with AQS only, the reduction rate of U(VI) is stable at approximately 30%. Compared with the control groups, the reduction rate of U(VI) of the experimental group with both AQS and bacteria is relatively higher. The reduction removal rate of U(VI) reaches 99.0% with AQS of 1.0 mmol/L after 4 d. This rate is more than the superposition of that of the control groups with either sole AQS or bacteria. AQS can be used as electron shuttle vector between U(VI) and electron donor to accelerate U(VI) reduction.
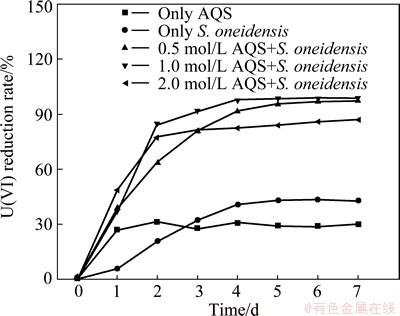
Fig. 3 U(VI) reduction rates of different redox systems
The addition of AQS in the experiments stimulates the U(VI) reduction to varying degrees. However, AQS concentration slightly influences the U(VI) reduction by S. oneidensis. The removal rates of U(VI) under AQS concentrations of 0.5, 1.0 and 2.0 mmol/L are 63.51%, 84.23% and 77.62%, respectively, after reduction for 2 d by microorganisms. Compared with the control group with bacteria only, the removal rate of U(VI) with addition of AQS increases by 43.01%, 63.73% and 57.12%, respectively. The stimulative role of AQS in the reduction of U(VI) by bacteria is positively correlated with the concentration of AQS in the range of 0.5-1.0 mmol/L. The catalytic role of AQS on the reduction of U(VI) weakens when the AQS concentration exceeds 1.0 mmol/L. The results are consistent with that of the reduction of azo dyes by Shewanella [18]. For most synthetic quinones, certain toxic effects are exerted on biological cells. S. oneidensis has limited tolerance to 2.0 mmol/L quinones with continuously increasing the AQS concentration. The electronic competition between AQS and U(VI) weakens the accelerative effects on U(VI) reduction.
3.3 Effect of pH value on U(VI) reduction
The pH value plays an important role in bacterial growth, and the biomass of bacteria greatly affects the U(VI) reduction. The effect of initial pH value on U(VI) reduction is plotted in Fig. 4. The pH value from 6.0 to 8.0 is more suitable for the growth of S. oneidensis. Under this condition, the reduction rate of U(VI) of each experimental group is more than 90% after 5 d, and the best removal efficiency is obtained at pH value of 7.0 with the removal rate of 95.21% after 5 d. However, 52.38% U(VI) is reduced at pH value of 4.0, and 76.42% U(VI) is reduced at pH value of 5.0 after 5 d.
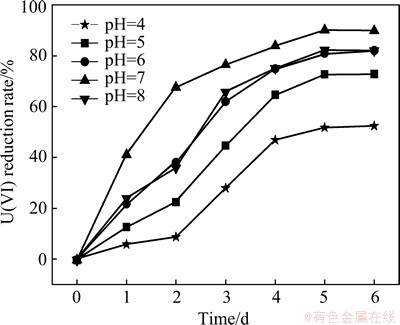
Fig. 4 Effect of pH value on U(VI) reduction
The results indicate that neutral environment is suitable for the growth of S. oneidensis, and the acidic environment of pH value less than 5.0 can inhibit the growth of microorganisms. However, S. oneidensis still induce certain reductive effects on U(VI) at pH values of 4.0-5.0. Consequently, the organism has high tolerance under acidic conditions.
3.4 Effects of ZVI on U(VI) reduction
In the past few years, the study on ZVI in pollution control and remediation of uranium-contaminated wastewater has become a hot spot. ZVI has several advantages such as rapid reaction rate, high oxidation– reduction potential, low price and readily available resources. One pair of control groups were prepared. One group was only added with ZVI, and the other group was only added with bacteria. The effects of ZVI dosages on U(VI) reduction were verified. Tests with both bacteria and ZVI were performed with ZVI dosages of 0.5, 1.0 and 2.0 g/L. In the groups mentioned above, 1.0 mmol/L AQS and 20.0 mg/L U(VI) were added into the cell culture medium.
The removal rates of U(VI) at different ZVI dosages are presented in Fig. 5. With 1 g/L ZVI, the reductive removal rate of U(VI) of the group with both ZVI and bacteria reaches 89.59% after 18 h, and the removal rate increases nearly 6 times that of the group with bacteria only. The removal rate of U(VI) reaches 95.01% after 24 h, whereas the reduction rate of U(VI) of the group with either sole ZVI or bacteria is less than 20% after 24 h. Additionally, 120 h is needed for the group with bacteria only to reduce about 95.01% U(VI). The reduction rates after 48 h in the redox system with both AQS and S. oneidensis are more than those of the controls with only ZVI or bacteria. This phenomenon illustrates that ZVI plays a distinctly stimulative role on reducing U(VI) by bacteria. Three reasons can be cited for the accelerative role of ZVI on U(VI) reduction. Firstly, under anaerobic condition, the hydrogen production via electrochemical corrosion of ZVI may assist the microbial growth. Secondly, the dissolved oxygen in the solution can be depleted with ZVI electrochemical reaction, maintaining anaerobic condition, which is beneficial for U(VI) reduction by S. oneidensis. Finally, the toxicity of U(VI) to the organism is weakened for the U(VI) reduction by ZVI.
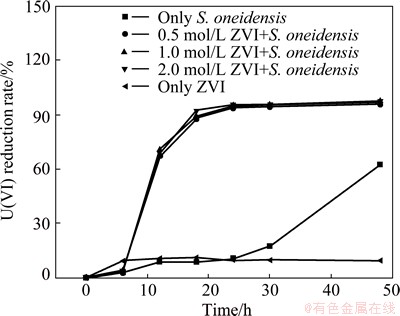
Fig. 5 Effect of ZVI on U(VI) reduction
The accelerative role of ZVI on the U(VI) reduction by bacteria is closely related to the amount of ZVI. The reductive removal rates of U(VI) at ZVI dosages of 0.5, 1.0 and 2.0 g/L reach 89.03%, 95.02% and 95.58%, respectively, after 24 h. Contrary to the group with 1.0 g/L ZVI, the removal rate of the group with 0.5 g/L ZVI decreases by 5.99%, and the removal rate of the group with 2.0 g/L ZVI increases by 0.56%. Consequently, when ZVI dosage ranges from 0.5 to 2.0 g/L, the higher the ZVI dosage, the greater the reduction rate of U(VI). In addition, when ZVI dosage ranges from 1.0 to 2.0 g/L, the reductive rate of U(VI) almost does not increase with increasing the ZVI dosage. This phenomenon may be due to the reaction of U(VI) reduction on the ZVI surface. Thus, the higher the ZVI dosage, the larger the surface of ZVI, which results in increased reductive rate.
3.5 Effects of coexisting metal ions of Cu2+, Cr6+, Mn2+ and Zn2+ on U(VI) reduction
The coexisting metal ions, such as Cu2+, Mn2+, Zn2+, influence the reduction efficiency of U(VI). In the experiments, 2.0 mmol/L Cu2+, Mn2+, Zn2+ and Cr6+ was sperately added into the redox systems of S. oneidensis and AQS. The effects of the above ions on the U(VI) reduction are shown in Fig. 6. The removal rates of U(VI) in the experimental groups with Cu2+, Mn2+, Zn2+ and Cr6+ are 5.69%, 40.52%, 92.82% and 47.25% after 72 h, respectively, and that of the control group without the above metal ions reaches 91.72% at the same time.
The results indicate that Zn2+ does not significantly affect the U(VI) reduction, but other metal ions of Cu2+, Mn2+ and Cr6+ inhibit the reduction. The inhibition of Cu2+ is the most prominent, followed by Mn2+. TANG et al [19] found that Cu2+ and Mn2+ also inhibited the reduction of Cr(VI) in the synergic removal of Cr(VI) in water by iron filings with microorganisms. The inhibition of Cu2+ may be due to the reductase activity of the protein on the cell membrane of S. oneidensis losing its ability of oxidation electron donor. This phenomenon is caused by the additional Cu2+ with the active center of the dehydrogenase protein from the initial respiratory chain, ultimately leading to the decrease of reductive rate of U(VI) [20,21]. The inhibition role of Cr6+ may be due to the strong oxidation ability of Cr6+, leading to the damage of the cell structure.
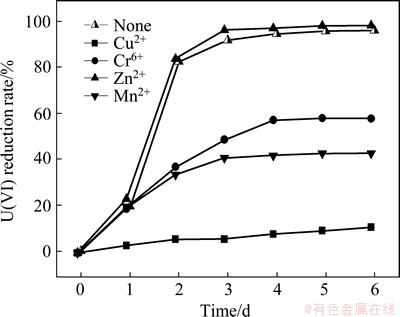
Fig. 6 Effect of different metal ions on U(VI) reduction
Mn2+ has also been reported to hinder the U(VI) reduction by microorganisms [22]. Mn2+ may be toxic to the organism and inhibit the bacterial growth. The removal effect of U(VI) is positively correlated with the biomass of bacteria, so the lower the biomass of the bacteria, the lower the removal rate of U(VI). In addition, the bacteria exhibit competitive selection of electron acceptor between Mn2+ and Cr6+, resulting in reduced reductive rate of U(VI).
3.6 Effects of coexisting anions on reduction of U(VI)
3.6.1 Effects of SO42- on U(VI) reduction
The reduction rate of U(VI) at different SO42- concentrations is presented in Fig. 7. The reduction rates of U(VI) of the groups with SO42- increase compared with that of the control groups without SO42-. The reduction rates of U(VI) reach 88.88%, 89.73%, 93.82% and 95.23% at SO42- concentrations of 0.2, 1.0, 2.0 and 5.0 mmol/L, respectively, after 3 d. The removal rates of the aforementioned groups remain stable at approximately 96% after 4 d. The reduction rates of the group without SO42- are 73.77% after 3 d and only 81.14% after 4 d.
The results indicate that SO42- can weakly promote the U(VI) reduction by S. oneidensis, and the reductive rate of U(VI) is positively correlated with SO42- concentrations varying from 0 to 5.0 mmol/L. S. oneidensis can utilize sulfate as the electron acceptor for energy metabolism, and SO42- anion can promote the activity of reductase in the oxidation- reduction process. Consequently, the reduction rates increase with increasing the addition of SO42-.
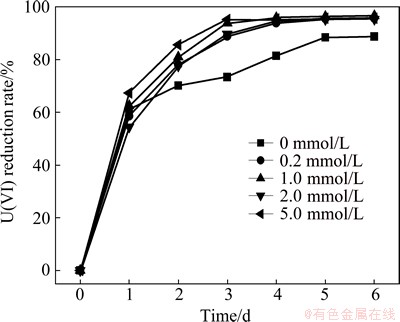
Fig. 7 Effect of SO42- on U(VI) reduction
3.6.2 Effects of NO3- on U(VI) reduction
The experimental and control groups were set to evaluate the effects of NO3- concentrations on the U(VI) reduction. In the experimental groups with NO3-, NH4Cl in the culture medium was replaced with NaNO3. However, NH4Cl was not replaced in the control group without NO3-. The reduction rates of U(VI) at different NO3- concentrations are presented in Fig. 8. When the NO3- concentration is 0.5 mmol/L, 96.92% U(VI) is reduced by S. oneidensis after 6 d. When the concentration of NO3- increases from 0.5 to 1.0 and 2.0 mmol/L, the reduction rate of U(VI) decreases from 96.92% to 10.07% and 7.11% after 6 d, respectively.
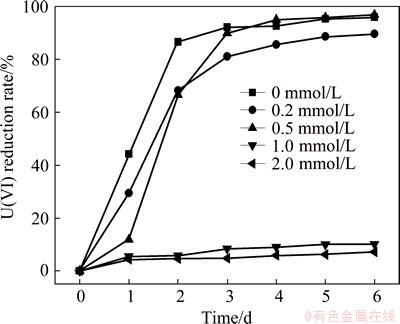
Fig. 8 Effect of NO3- on U(VI) reduction
However, the reduction rate of U(VI) of the control group without NO3- ion reaches 96.07% after 6 d.
The results show that NO3- has no obvious inhibitive influence on the reduction of U(VI) in the concentration range of 0-0.5 mmol/L. However, NO3- inhibits the reductive process when the concentration of NO3- is more than 1.0 mmol/L. This phenomenon is due to the fact that N5+ in the oxidation state from NO3- has powerful oxidative character. Moreover, NO3- (>1.0 mmol/L) probably competes for the free electron with U(VI) during the reduction, resulting in adverse reductive effects. However, NO3- (<0.5 mmol/L) can be used by the cell through nitrification to promote its growth and reproduction.
4 Morphological analyses on reductive products of U(VI)
XPS was performed to analyze the valence state of uranium to the cell surface and characterize the reductive products of U(VI). Figure 9 shows two obvious peaks. One peak is in the orbit of U 4f7/2 with binding energy of 380-382 eV, and the other peak is in the orbit of U 4f5/2 in the 392-393 eV region. According to the Analytical Handbook of XPS [23], the peak at 4f7/2 can be attributed to UO2 [U(IV)] with binding energy of 380.3±0.4 eV and UO3 [U(VI)] with binding energy of 381.6±0.3 eV, and the ratio of UO2 to UO3 is around 8:3. The other peak at 4f5/2 can be attributed to U3O8 in the 392 eV region and UO3 [U(VI)] in the 392.65 ±0.15 eV region, and the ratio of U3O8 to UO3 is around 5:1. These results demonstrate that uranium is on the cell surface with U(IV) and U(VI) forms. The majority of U(VI) is induced to stable U(IV) by bacteria, and U(VI) on the cell is probably due to the biosorption of bacteria.
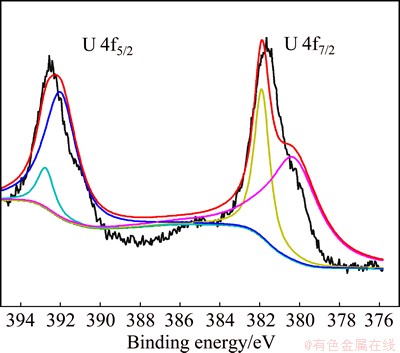
Fig. 9 Characteristics of uranium on cell surface
5 Conclusions
1) In the presence of AQS, U(VI) can be reduced effectively in anaerobic environment by S. oneidensis. The reduction rate of U(VI) reaches 99.0% at 30 °C after 96 h with the pH value of 7.0 and 1.0 mmol/L AQS. The effective concentration of AQS as the accelerator for U(VI) bioreduction is 0.5-1.0 mmol/L.
2) ZVI promotes the reduction of U(VI) in the system of AQS and S. oneidensis, and the reduction rate improves with increasing the ZVI dosage in the range of 0-2.0 g/L.
3) The coexistence of 2.0 mmol/L ions, such as Cu2+, Mn2+ and Cr6+, inhibits the U(VI) reduction. Moreover, 2.0 mmol/L Zn2+ and less than 0.5 mmol/L NO3- exhibit no significant effect on the U(VI) reduction. Additionally, less than 5.0 mmol/L SO42- slightly promotes the U(VI) reduction.
4) Uranium on the surface of bacteria is deposited in the U(IV) or U(VI) form. The majority of U(VI) in the solution forms stable UO2.
References
[1] Rodgher S, Azevedo d H, Ferrari C R, ROQUE C V RONQUI L B. Evaluation of surface water quality in aquatic bodies under the influence of uranium mining (MG, Brazil) [J]. Environmental Monitoring and Assessment, 2013, 185(3): 2395-2406.
[2] CHEN Hua-bai, XIE Shui-bo, LIU Jin-xiang, XIAO Shi-hong, ZENG Tao-tao, LING Hui, WANG Jin-song. Characteristics and mechanism of uranium(VI) absorbed by anaerobic granular sludge [J]. The Chinese Journal of Nonferrous Metals, 2014, 24(9): 2418-2425. (in Chinese)
[3] Fredrickson J K, Romine M F, Beliaev A S, AuchtunG J M. Towards environmental systems biology of Shewanella [J]. Nature Reviews Microbiology, 2008, 6(8): 592-603.
[4] Liu C, Zachara J M, Zhong L,Heald S M,Wang Z, Jeon B H,Fredrickson J K. Microbial of reduction intragrain U(VI) in contaminated sediment [J]. Environmental Science and Technology, 2009, 43(13): 4928-4933.
[5] LUAN F, BURGOS W D. Bioreduction of nitrobenzene, natural organic matter, and hematite by Shewanella putrefaciens CN32 [J]. Environmental Science and Technology, 2010, 44(12): 184-190.
[6] Icopini G A, Lack J G, Hersman L E, Neu M P, Boukhalfa H. Plutonium(V/VI) reduction by the metal-reducing bacteria Geobacter metallireducens GS-15 and Shewanella oneidensis MR-1[J]. Applied and Environmental Microbiology, 2009, 75(11): 3641-3647.
[7] DOLLHOPF M E, NEALSON K H, SIMON D M, LUTHER G W. Kinetics of Fe (III) and Mn (IV) reduction by the black sea strain of Shewanella putrefaciens using in situ solid state voltammetric Au/Hg electrodes [J]. Marine Chemistry, 2000, 70(1-3): 171-180.
[8] CROSBY H, RODEN E. The mechanisms of iron isotope fractionation produced during dissimilatory Fe(III) reduction by Shewanella putrefaciens and Geobacter sulfurreducens [J]. Geobiology, 2007, 5(2): 169-189.
[9] Lovley D R, Coates J D, Bluntharris E L. Humic substances as electron acceptors for microbial respiration
[10] XIE Shui bo, ZHANG Ya ping, LIU Jin xiang, LIU Ying jiu, LI Shi you, WANG Jin song, LIU Hai yan. Characteristics of reducing U(VI) by Shewanella putrefaciens in presence of anthraguinone 2 sulfonate (AQS) [J]. The Chinese Journal of Nonferrous Metals, 2012, 22(11): 3285-3291. ( in Chinese )
[11] Zhang F, Wu W M, Parker J C, Mehlhorn T,Kelly S D, Kemner K M, Zhang G, Schadt C, Brooks S C,Criddle C S,Watson D B,Jardine P M. Kinetic analysis and modeling of oleate and ethanol stimulated uranium(VI) bio-reduction in contaminated sediments under sulfate reduction conditions [J]. Journal of Hazardous Materials, 2010, 183(1-3): 482-489.
[12] Guo J B, Lian J, Xu Z F, Xi Z H, Yang J L, Jefferson W, Liu C, Li Z X, Yue L.Reduction of Cr(VI) by Escherichia coli BL21 in the presence of redox mediators [J]. Bioresource Technology, 2012, 123(3): 713-716.
[13] XU Zhi cheng, HONG Yi guo, LUO We, XU Mei ying, SUN Guo ping. The effects of the humic substances on azo reduction by Shewanella spp [J]. Acta Microbiologica Sinica, 2006, 46(4): 591-597. ( in Chinese )
[14] Cai P J, Xiao X, He Y R, Li W W,Chu J,Wu C,He M X,Zhang Z,Sheng G P,Lam M H,Xu F,Yu H Q. Anaerobic biodecolorization mechanism of methyl orange by Shewanella oneidensis MR-1 [J]. Applied Microbiology and Biotechnology, 2012, 93(4): 1769-1776.
[15] Xiao X, Xu C C, Wu Y M, Cai P J,LI W W, DU D L, YU H Q. Biodecolorization of naphthol green B dye by Shewanella oneidensis MR-1 under anaerobic conditions [J]. Bioresource Technology, 2012, 110(4): 86-90.
[16] Gralnick J A, Vali H, Lies D P,Newman D K. Extracellular respiration of dimethyl sulfoxide by Shewanella oneidensis strain MR-1 [J]. Proceedings of the National Academy of Sciences of the United States of America, 2006, 103(12): 4669-4674.
[17]  A, Luna-Velasco A, Field J A, sierrA-ALVAREZ R. Toxicity of uranium to microbial communities in anaerobic biofilms [J]. Water, Air and Soil Pollution, 2012, 223(7): 3859-3868.
A, Luna-Velasco A, Field J A, sierrA-ALVAREZ R. Toxicity of uranium to microbial communities in anaerobic biofilms [J]. Water, Air and Soil Pollution, 2012, 223(7): 3859-3868.
[18] XU Zhi cheng, HONG Yi guo, LUO We, CHEN Xing juan, SUN Guo ping. Anaerobic humus respiration by Shewanella cinica D14T [J]. Acta Microbiologica Sinica, 2006, 46(6): 973-978. (in Chinese)
[19] TANG Jie, WANG Zhuo-xing, XU Xin-hua. Removal of Cr(VI) by iron filings with microorganisms to recover iron reactivity [J]. Environmental Science, 2013, 34(7): 2650-2657. (in Chinese)
[20] Beliaev A S, Saffarini D A, McLaughlin J L, Hunnicutt D. MtrC, an outer membrane decahaem c cytochrome required for metal reduction in Shewanella putrefaciens MR-1 [J]. Molecular Microbiology, 2001, 39(3): 722-730.
[21] FERNANDEZ V M, RUA M L, REYES P, CAMMACK R, HATCHIKIAN E C. Inhibition of desulfovibrio gigas hydrogenase with copper salts and other metal ions [J]. European Journal of Biochemistry, 1989, 185(2): 449-454.
[22] VERRAMANI H, SCHOFIELD E J, SHARP J O, SUVOROVA E I, ULRICH K U, MEHTA A, GIAMMAR D E, BARGAR J R, BERNIER LATMANI R. Effect of Mn(II) on the structure and reactivity of biogenic uraninite [J]. Environmental Science and Technology, 2009, 43(17): 6541-6547.
[23] WAGNER C D, RIGGS W M, DAVIS L E, MOULDER J F. Handbook of X-ray photoelectron spectroscopy [M]. Minnesota: Perkin-Elmer Corp, 1992: 42-77.
刘金香1,谢水波1,2,王永华1,刘迎九1,蔡萍莉1,熊 芬1,王文涛1
1. 南华大学 污染控制与资源化技术湖南省高校重点实验室,衡阳 421001;
2. 南华大学 铀矿冶生物技术国防重点学科实验室,衡阳 421001
摘 要:在厌氧环境下,以蒽醌-2-磺酸钠(AQS)作为腐殖质模式物,对奥奈达希瓦氏菌(Shewanella oneidensis, S. oneidensis)还原U(VI)的效果与主要影响因素进行研究。结果表明:在30 °C,当pH值为7.0,AQS为1.0 mmol/L,反应96 h后,S. oneidensis对U(VI)的去除率达到99.0%。AQS浓度为0.5~1.0 mmol/L时,其能有效促进U(VI)的还原,当AQS浓度超过2.0 mmol/L时,其对U(VI)的还原促进作用明显减小。Cu2+、Cr6+和Mn2+对U(VI)的还原均表现出明显的抑制作用,而Zn2+对U(VI) 的还原影响较小。NO3-离子浓度低于0.5 mmol/L时,其对U(VI)的还原基本没有影响,而当NO3-离子浓度高于1.0 mmol/L时,其对U(VI)的还原存在明显的抑制作用。当SO42-离子浓度低于5.0 mmol/L时,其对U(VI)的还原有微弱的促进作用。在AQS+S. oneidensis+ U(VI)还原体系中投加零价铁(ZVI),能快速促进U(VI)的还原,且U(VI)的还原率与ZVI的投加量(0~2.0 mg/L)呈正相关。XPS分析结果表明:菌体表面沉积U(VI)和U(IV)两种价态的U元素,反应后的铀主要形成稳定的UO2。
关键词:U(VI);蒽醌-2-磺酸钠(AQS);奥奈达希瓦氏菌;XPS
(Edited by Mu-lan QIN)
Foundation item: Projects (11175081, 11475080) supported by the National Natural Science Foundation of China; project (13JJ3078) supported by the Natural Science Foundation of Hunan Province, China; Project (14k083) supported by the Innovation Platform Open Fund Project of University in Hunan Province, China
Corresponding author: Wen-tao WANG; Tel: +86-734-8281603; E-mail: xiesbmr@263.ne
DOI: 10.1016/S1003-6326(15)64080-8

