文章编号:1004-0609(2017)-01-0138-07
低品位氧化锌矿硫酸铵的焙烧过程及其反应机理
邵鸿媚,申晓毅,朱慧婷,翟玉春
(东北大学 冶金学院,沈阳 110819)
摘 要:采用热分析研究低品位氧化锌矿与硫酸铵的焙烧过程,考察铵矿比、焙烧温度、焙烧时间对锌提取率的影响;采用XRD分析揭示氧化锌矿中有价组元在焙烧中的反应过程和相变行为;讨论焙烧过程中发生的化学反应。结果表明:合适的反应条件为铵矿比1.4:1、焙烧温度475 ℃、焙烧时间60 min;硫酸铵焙烧低品位氧化锌矿是多相反应,ZnO和ZnCO3与硫酸铵反应先生成(NH4)2Zn(SO4)2,升温后分解得到ZnSO4;铁氧化物与硫酸铵反应先生成(NH4)3Fe(SO4)3,分解得到NH4Fe(SO4)2;CaCO3和PbO在400 ℃以上焙烧时转化为硫酸钙和硫酸铅。
关键词:低品位氧化锌矿;硫酸铵;反应过程;相变行为
中图分类号:TF813 文献标志码:A
氧化锌矿是重要的锌资源,储量丰富。随着硫化锌矿资源的日渐枯竭,氧化锌矿的开发利用势在必行[1-4]。多年来,各国已在中低品位氧化锌矿的利用方面开展了很多研究,主要有火法和湿法两类[3-4]。传统火法工艺主要为烟化富集和高温直接还原氧化锌制备粗锌或氧化锌,工序多、能耗高、锌收率较低,逐步被湿法工艺所取代[5-6]。湿法工艺包括酸法、碱法和微生物浸出法。酸法处理氧化锌矿是目前研究最多、应用也最为广泛的方法,又分常压和高压酸浸,包括浸出、净化、电积等工序,浸出过程需严格控制以防生成硅胶影响物料过滤性能,物料腐蚀性强[7-9];碱法工艺有氨法和氢氧化钠法,氨法工艺以铵盐或添加氨的方法浸出锌,再电解制锌或沉淀制备碱式碳酸锌,煅烧制氧化锌,工艺对设备要求高,需密闭处理[10-13];氢氧化钠法可同时浸出矿石中锌、铅、硅,浸出效率高,不受铁钙影响,如何有效分离锌、铅、硅尚待优化[13-14]。微生物浸出法尚处在实验室研究阶段[15]。氧化锌矿物相复杂,嵌布粒度细,难以用选矿的方法富集[1, 16]。本文作者将火法和湿法工艺结合,提出一条综合利用低品位氧化锌矿的工艺路线。采用硫酸铵焙烧低品位氧化锌矿提锌,提锌渣转化法提铅,提铅渣碱熔融提硅,最后富集铁。整个过程反应条件温和,综合提取利用低品位氧化锌矿中有价组分锌、铅、硅、铁并制备成产品,化工原料硫酸铵和氢氧化钠循环使用。在此,主要考察硫酸铵焙烧低品位氧化锌矿的过程及其反应机理。
1 实验
1.1 实验原料和仪器
低品位氧化锌矿产自云南某地,硫酸铵为工业级,去离子水实验室自制。实验仪器有自制竖式焙烧炉和智能控温仪、电热恒温水浴锅、搅拌器、真空抽滤机。
1.2 实验方法
将低品位氧化锌矿破碎、磨细后与硫酸铵混合均匀。称取100 g混合物料置于刚玉坩埚中,将坩埚放入焙烧炉,在设定的温度制度下焙烧,待焙烧结束后,随炉冷取样。物料趁热加水经80 ℃溶出1 h,趁热过滤,滤饼淋洗3次,得到含硫酸锌的溶液和滤饼。滤饼烘干,检测两者中的锌含量,并计算其提取率。
氧化锌矿中ZnO、Fe2O3、PbO和CaO与硫酸铵按化学计量比恰好完全反应生成正盐消耗的硫酸铵的量计为1,此时铵矿比为1:1,硫酸铵过量10%,计为铵矿比1.1:1。
采用D/MAX-2500PC型X射线衍射仪表征样品晶态(Cu Kα1, λ=0.15406 nm),用Hitachi SU8010型扫描电镜观测样品形貌。
2 结果和讨论
2.1 低品位氧化锌矿的表征
图1所示为低品位氧化锌矿的XRD谱和SEM像。表1所列为低品位氧化锌矿的主要化学成分定量分析结果。由图1可见,低品位氧化锌矿的主要物相成分为菱锌矿ZnCO3、石英SiO2、赤铁矿Fe2O3、CaCO3,此外还有硅酸锌Zn2SiO4、PbO及CaSO4·2H2O,矿相复杂。SiO2含量高达48.77%,其次为CaO和ZnO,分别为8.96%和8.71%。采用硫酸铵焙烧,与CaO结合的SO42-无法循环,造成材料浪费,这与酸浸类似。氧化锌矿颗粒表面粗糙,粒度不均匀。
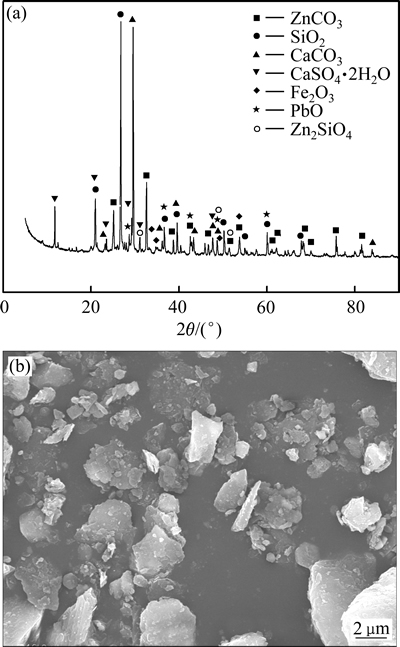
图1 低品位氧化锌矿的XRD谱和SEM像
Fig. 1 XRD pattern(a) and SEM image(b) of low grade zinc oxide ore
表1 低品位氧化锌矿主要化学成分定量分析
Table 1 Quantitative analysis of main chemical compositions in low grade zinc oxide ore (mass fraction, %)

2.2 铵矿比对锌提取率的影响
图2所示为在焙烧温度450 ℃、焙烧时间60 min条件下铵矿比与锌提取率的关系曲线。由图2可见,随铵矿比增加,锌提取率增加。铵矿比从1:1到1.3:1,锌提取率从40.10%提高到93.33%,在铵矿比1.4:1以后,锌提取率变化不大。这是由于硫酸铵不足时氧化锌矿反应不充分,增加硫酸铵的用量,氧化锌矿颗粒与硫酸铵粉体接触充分,促进反应进行。铵矿比1.4:1时锌提取率达到96.25%,本实验中铵矿比选择为1.4:1。
2.3 焙烧温度对锌提取率的影响
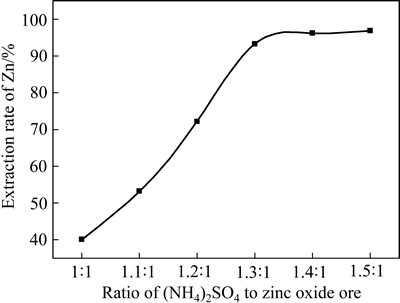
图2 铵矿比与锌提取率的关系
Fig. 2 Relationship between ratio of (NH4)2SO4 to zinc oxide ore and extraction rate of Zn
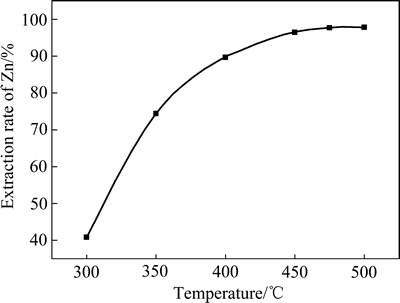
图3 焙烧温度与锌提取率的关系
Fig. 3 Relationship between roasting temperature and extraction rate of Zn
图3所示为在铵矿比1.4:1、焙烧时间60 min条件下焙烧温度与锌提取率的关系曲线。由图3可见,随焙烧温度的增加锌提取率增加。焙烧温度从300 ℃升至475 ℃,锌提取率从40.83%增加到97.69%。再升高焙烧温度,锌提取率基本稳定。这是由于随着焙烧温度升高,硫酸铵分解得到液相硫酸氢铵,硫酸氢铵分解可得硫酸,固-固相反应转变为液-固相反应,反应阻力降低,促进反应进行,实验选择焙烧温度475 ℃。
2.4 焙烧时间对锌提取率的影响
图4所示为铵矿比1.4:1、焙烧温度475 ℃条件下焙烧时间与锌提取率的关系。由图4可见,随焙烧时间增加,锌提取率增加。焙烧时间60 min,锌提取率最高为98.13%,再延长焙烧时间,锌提取率反而略有降低。这是由于反应初期进行的主要是锌化合物与硫酸铵及分解产物的反应,60 min后,铁氧化物与硫酸铵及分解产物的反应加强,铁提取率提高,增加净化除杂负担。实验选择焙烧时间60min。
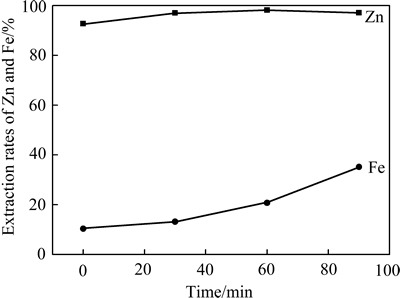
图4 焙烧时间与锌、铁提取率的关系
Fig. 4 Relationship between roasting time and extraction rates of Zn and Fe
2.5 焙烧过程反应机理
图5所示为硫酸铵焙烧低品位氧化锌矿的TG-DTA曲线。曲线上在50、290、330、400及530 ℃上出现多个吸热峰。为分析硫酸铵焙烧氧化锌矿的反应过程,采用XRD分析了不同温度焙烧物料的物相。图6所示为铵矿比1.4:1条件下不同温度焙烧物料的XRD谱。图6(a)~(e)所示分别对应焙烧温度250、300、350、400和475 ℃。可见,250 ℃时,焙烧物料物相为SiO2、ZnCO3、(NH4)2SO4和CaCO3,表明在250 ℃反应尚不明显。300 ℃时,焙烧物料物相为SiO2、ZnCO3和CaCO3,相比250 ℃的XRD谱可知,SiO2、CaCO3峰不变,ZnCO3和(NH4)2SO4峰变弱。表明300 ℃时ZnCO3与(NH4)2SO4已开始反应。350 ℃时,焙烧物料物相为SiO2、Zn2SiO4、(NH4)3Fe(SO4)3、(NH4)2Zn(SO4)2·6H2O和CaCO3,ZnCO3消失,(NH4)2SO4未分解完全,CaCO3仍存在,Zn2SiO4为原矿自带。生成的新相(NH4)3Fe(SO4)3、(NH4)2Zn(SO4)2·6H2O表明ZnCO3、铁氧化物与(NH4)2SO4反应优先生成硫酸复盐,而非直接得到Fe2(SO4)3和ZnSO4。400 ℃时,焙烧物料物相为SiO2、(NH4)2Zn(SO4)2、CaCO3和(NH4)3Fe(SO4)3,与350 ℃的物料图谱相比,CaCO3不变,(NH4)2SO4消失,Zn2SiO4和(NH4)2Zn(SO4)2·6H2O晶相消失,得到(NH4)2Zn(SO4)2。表明(NH4)2SO4已分解完全,Zn2SiO4与(NH4)2SO4反应生成(NH4)2Zn(SO4)2。475 ℃焙烧物料物相为SiO2、CaSO4、PbSO4、(NH4)2Zn(SO4)2、NH4Fe(SO4)2和ZnSO4,与400 ℃时的物料图谱相比,CaCO3和PbO消失,产生新相ZnSO4、CaSO4和PbSO4。表明温度升至475 ℃,CaCO3和PbO转化为CaSO4和PbSO4,(NH4)2Zn(SO4)2开始分解生成ZnSO4,(NH4)3Fe(SO4)3脱铵得到NH4Fe(SO4)2,继续升温可得到Fe2(SO4)3。
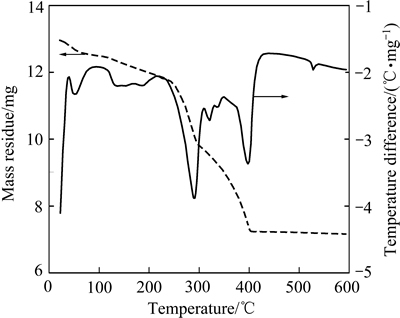
图5 硫酸铵焙烧低品位氧化锌矿的TG-DTA曲线
Fig. 5 TG-DTA curves of low grade zinc oxide ore roasted by ammonium sulfate
可见,TG-DTA曲线上,50 ℃的吸热峰为吸附水脱除;290 ℃的吸热峰涉及ZnCO3和硫酸铵的分解、锌和铁化合物与硫酸铵的反应等;330 ℃的吸热峰涉及碳酸盐的分解;400 ℃的吸热峰涉及硫酸锌铵分 解、硫酸铁铵分解、Zn2SiO4与硫酸铵及其分解产物反应、碳酸钙和氧化铅转化为硫酸钙和硫酸铅的反应;530 ℃的吸热峰为NH4Fe(SO4)2分解为Fe2(SO4)3的吸热峰。
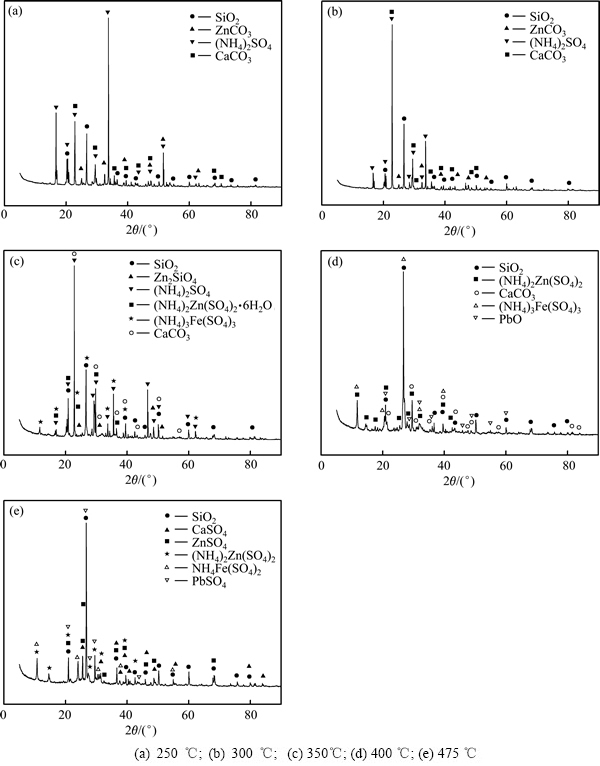
图6 不同温度焙烧物料的XRD谱
Fig. 6 XRD patterns of specimens roasted at different temperatures
综上所述,硫酸铵焙烧低品位氧化锌矿的反应过程可以简述为ZnO和ZnCO3与(NH4)2SO4优先反应,生成硫酸锌铵复盐,复盐吸水可生成(NH4)2Zn(SO4)2·6H2O。铁氧化物与硫酸铵反应生成(NH4)3Fe(SO4)3,继续升温脱铵生成NH4Fe(SO4)2,要得到Fe2(SO4)3需要更高的温度。400 ℃以上时,硅酸锌与硫酸铵及分解产物反应生成硫酸锌铵,CaCO3和PbO转化为CaSO4和PbSO4,(NH4)2Zn(SO4)2开始分解生成ZnSO4。475 ℃焙烧物料中主要物相为石英态SiO2、ZnSO4、(NH4)2Zn(SO4)2、NH4Fe(SO4)2、CaSO4和PbSO4。ZnSO4和(NH4)2Zn(SO4)2均可溶于水,经水溶实现Zn与Si、Pb的分离,NH4Fe(SO4)2也进入溶液。硫酸铵焙烧低品位氧化锌矿的反应过程复杂,根据XRD谱和参考文献[17-20],焙烧过程发生的主要化学反应可归纳如下:
(NH )2SO4=NH4HSO4+NH3↑ (1)
ZnCO3=ZnO+CO2↑ (2)
ZnCO3+2(NH4)2SO4=(NH4)2Zn(SO4)2+2NH3↑+H2O↑+CO2↑ (3)
ZnO+2(NH4)2SO4=(NH4)2Zn(SO4)2+2NH3↑+H2O↑ (4)
Zn2SiO4+4(NH4)2SO4=2(NH4)2Zn(SO4)2+4NH3↑+2H2O↑+SiO2↑ (5)
Fe2O3+6(NH4)2SO4=2(NH4)3Fe(SO4)3+6NH3↑+3H2O↑ (6)
Fe2O3+6NH4HSO4=2(NH4)3Fe(SO4)3+3H2O↑ (7)
(NH4)3Fe(SO4)3=NH4Fe(SO4)2+2NH3↑+H2O↑+SO3↑ (8)
(NH4)2Zn(SO4)2=ZnSO4+2NH3↑+H2O↑+SO3↑ (9)
NH4HSO4=H2SO4+NH3↑ (10)
CaCO3+H2SO4=CaSO4+H2O↑+CO2↑ (11)
PbO+H2SO4=PbSO4+H2O↑ (12)
2.6 提锌渣的表征
图7所示为提锌渣的XRD谱和SEM像。由图7可见,提锌渣主要物相为石英态SiO2、CaSO4、PbSO4、CaSO4·2H2O和较难反应的Fe3O4。提锌渣颗粒粒度范围大,表面粗糙。
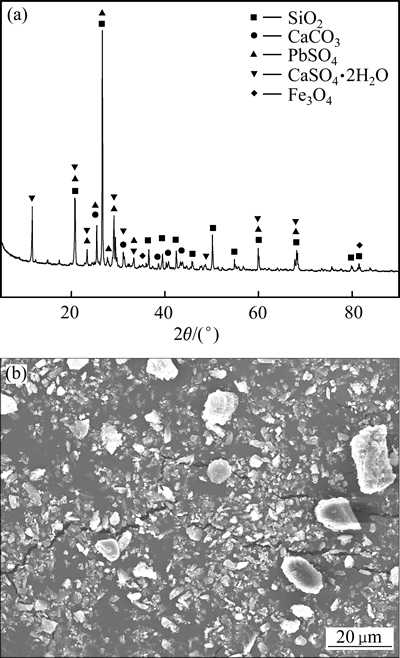
图7 提锌渣的XRD谱和SEM像
Fig. 7 XRD pattern(a) and SEM image(b) of leaching residue
3 结论
1) 硫酸铵焙烧低品位氧化锌矿的合适反应条件为:铵矿比1.4:1、焙烧温度475 ℃、焙烧时间60 min,在此条件下锌提取率达到98%。
2) 硫酸铵焙烧低品位氧化锌矿反应过程复杂,随温度升高,ZnO和ZnCO3与硫酸铵反应先生成硫酸锌铵,升温分解得到ZnSO4。铁氧化物与硫酸铵反应先生成(NH4)3Fe(SO4)3,脱铵得到NH4Fe(SO4)2。400 ℃以上时,CaCO3转化为CaSO4,PbO转化为PbSO4,石英态SiO2不参加反应。(NH4)2Zn(SO4)2和ZnSO4均可溶于水,经水溶实现Zn与Si、Pb、Ca的分离。
REFERENCES
[1] 刘智勇, 刘志宏, 曹志阎, 李启厚, 杨天足. 硅锌矿在(NH4)2SO4-NH3-H2O体系中的浸出机理[J]. 中国有色金属学报, 2011, 21(11): 2929-2935.
LIU Zhi-yong, LIU Zhi-hong, CAO Zhi-yan, LI Qi-hou, YANG Tian-zu. Leaching mechanism of willemite in (NH4)2SO4-NH3- H2O system[J]. The Chinese Journal of Nonferrous Metals, 2011, 21(11): 2929-2935.
[2] 贺山明, 王吉坤. 氧化锌矿冶金处理的研究现状[J]. 矿冶, 2010, 19(3): 58-65.
HE Shan-ming, WANG Ji-kun. Review on research of metallurgical processing of zinc oxide ores[J]. Mining and Metallurgy, 2010, 19(3): 58-65.
[3] 夏志美, 陈艺峰, 王宇菲, 王 超, 李 丽. 低品位氧化锌矿的湿法冶金研究进展[J]. 湖南工业大学学报, 2010, 24(6): 9-13.
XIA Zhi-mei, CHEN Yi-feng, WANG Yu-fei, WANG Chao, LI Li. Development of treating low-grade zinc oxide ore by hydrometallurgy[J]. Journal of Hunan University of Technology, 2010, 24(6): 9-13.
[4] 丰奇成, 文书明, 柏少军, 吕梦阳, 陈赐云. 低品位难处理氧化锌矿综合利用现状[J]. 矿产综合利用, 2013(1): 4-8.
FENG Qi-cheng, WEN Shu-ming, BAI Shao-jun,  Meng-yang, CHEN Ci-yun. Comprehensive utilization status of low-grade and refractory zinc oxide ores[J]. Multipurpose Utilization of Mineral Resources, 2013(1): 4-8.
Meng-yang, CHEN Ci-yun. Comprehensive utilization status of low-grade and refractory zinc oxide ores[J]. Multipurpose Utilization of Mineral Resources, 2013(1): 4-8.
[5] DOU Ai-chun, YANG Tian-zu, YANG Ji-xing, WU Jiang-hua, WANG An. Leaching of low grade zinc oxide ores in Ida2--H2O system[J]. Transactions of Nonferrous Metals Society of China, 2011, 21(11): 2548-2553.
[6] MORADI S, MONHEMIUS A J. Mixed sulphide-oxide lead and zinc ores: Problems and solutions[J]. Minerals Engineering, 2011, 24(10): 1062-1076.
[7] HE Shan-ming, WANG Ji-kun, YAN Jiang-fei. Pressure leaching of synthetic zinc silicate in sulfuric acid medium[J]. Hydrometallurgy, 2011, 108(3/4): 171-176.
[8] 贺山明, 王吉坤, 汪金良, 黎 阳, 干 磊, 熊辉辉. 硅酸锌加压硫酸浸出的热力学分析与实验研究[J]. 过程工程学报, 2014, 14(6): 930-936.
HE Shan-ming, WANG Ji-kun, WANG Jin-liang, LI Yang, GAN Lei, XIONG Hui-hui. Thermodynamic analysis and experiment on pressure leaching of zinc silicate with sulfuric acid[J]. The Chinese Journal of Process Engineering, 2014, 14(6): 930-936.
[9] QIN Wen-qing, LI Wei-zhong, LAN Zhuo-yue, QIU Guan-zhou. Simulated small-scale pilot plant heap leaching of low-grade oxide zinc ore with integrated selective extraction of zinc[J]. Minerals Engineering, 2007, 20(7): 694-700.
[10] DING Zhi-ying, YIN Zhou-lan, HU Hui-ping, CHEN Qi-yuan. Dissolution kinetics of zinc silicate (hemimorphite) in ammoniacal solution[J]. Hydrometallurgy, 2010, 104(2): 201-206.
[11] 夏志美, 杨声海, 唐谟堂, 杨天足, 刘志宏, 唐朝波, 何 静, 邓小林. MACA体系中循环浸出低品位氧化锌矿制备电解锌[J]. 中国有色金属学报, 2013, 23(11): 3455-3461.
XIA Zhi-mei, YANG Sheng-hai, TANG Mo-tang, YANG Tian-zu, LIU Zhi-hong, TANG Chao-bo, HE Jing, DENG Xiao-ling. Cycle leaching of low grade zinc oxide ores in MACA system for preparing zinc[J]. [J]. The Chinese Journal of Nonferrous Metals, 2013, 23(11): 3455-3461.
[12] YIN Zhou-lan, DING Zhi-ying, HU Hui-ping, LIU Kui, CHEN Qi-yuan. Dissolution of zinc silicate (hemimorphite) with ammonia-ammonium chloride solution[J]. Hydrometallurgy, 2010, 103(1/4): 215-220.
[13] CHEN Ai-liang, ZHAO Zhong-wei, JIA Xi-jun, LONG Shuang, HUO Guang-sheng, CHE Xing-yu. Alkaline leaching Zn and its concomitant metals from refractory hemimorphite zinc oxide ore[J]. Hydrometallurgy, 2009, 97(3/4): 228-232.
[14] FENG Lin-yong, YANG Xian-wan, SHEN Qing-feng, XU Ming-li, JIN Bing-jie. Pelletizing and alkaline leaching of powdery low grade zinc oxide ores[J]. Hydrometallurgy, 2007, 89(3/4): 305-310.
[15] 申晓毅, 孙 毅, 宋继强, 翟玉春. 硫酸铵低温焙烧中低品位氧化锌矿[J]. 材料研究学报, 2012, 26(4): 396-401.
SHEN Xiao-yi, SUN Yi, SONG Ji-qiang, ZHAI Yu-chun. Low grade zinc oxide ore by low temperature roasting using (NH4)2SO4[J]. Chinese Journal of Materials Research, 2012, 26(4): 396-401.
[16] 宋 龑, 刘全军, 常富强. 氧化锌矿的浮选现状与研究进展[J]. 矿冶, 2012, (2): 19-22.
SONG Yan, LIU Quan-jun, CHANG Fu-qiang. Status and research progress of zinc oxide ore flotation[J]. Mining and Metallurgy, 2012, 21(2): 19-22.
[17] 辛海霞, 吴 艳, 刘少名, 翟玉春. 高铁铝土矿与硫酸氢铵混合焙烧工艺[J]. 中国有色金属学报, 2014, 24(3): 808-813.
XIN Hai-xia, WU Yan, LIU Shao-ming, ZHAI Yu-chun. Roasting process of mixture of high ferric bauxite and ammonium bisulfate[J]. The Chinese Journal of Nonferrous Metals, 2014, 24(3): 808-813.
[18] 石剑锋, 王志兴, 胡启阳, 郭华军, 李新海, 彭文杰. 硫酸氢铵硫酸化焙烧法红土镍矿提取镍钴[J]. 中国有色金属学报, 2013, 23(2): 510-515.
SHI Jian-feng, WANG Zhi-xing, HU Qi-yang, GUO Hua-jun, LI Xin-hai, PENG Wen-jie. Recovery of nickel and cobalt from nickel laterite ore by sulfation roasting method using ammonium bisulfate[J]. The Chinese Journal of Nonferrous Metals, 2013, 23(2): 510-515.
[19] SONG X F, ZHAO J C, LI Y Z, SUN Z, YU J G. Thermal decomposition mechanism of ammonium sulfate catalyzed by ferric oxide[J]. Frontiers of Chemical Science and Engineering, 2013, 7(2): 210-217.
[20] 辛海霞, 吴 艳, 刘少名, 翟玉春. 硫酸铵-高铁铝土矿焙烧法提取铝、铁[J]. 矿产保护和利用, 2013(4): 37-40.
XIN Hai-xia, WU Yan, LIU Shao-ming, ZHAI Yu-chun. Recovery of aluminum and iron from high-iron bauxite by roasting method using ammonium sulfate[J]. Conservation and Utilization of Mineral Resources, 2013(4): 37-40.
Process and reaction mechanism of roasting low grade zinc oxide ore with (NH4)2SO4
SHAO Hong-mei, SHEN Xiao-yi, ZHU Hui-ting, ZHAI Yu-chun
(School of Metallurgy, Northeastern University, Shenyang 110819, China)
Abstract: The process of roasting low grade zinc oxide ore with ammonium sulfate was studied by thermal analysis, and the influences of ratio of ammonium sulfate to zinc oxide ore, roasting temperature and roasting time on the extraction ratio of zinc oxide were investigated. The XRD analysis was adopted to illuminate the reaction process and the phases transformation behavior of the valuable compositions in zinc oxide ore. Finally, the chemical reactions occured in roasting process were discussed. The results show that appropriate reaction conditions are as follows, ratio of ammonium sulfate to zinc oxide ore of 1.4:1, roasting temperature of 475 ℃ and roasting time of 60 min. Roasting low grade zinc oxide ore using ammonium sulfate is a multiphase process. Firstly, ZnO and ZnCO3 react with ammonium sulfate to produce (NH4)2Zn(SO4)2, which then decomposes into ZnSO4. Similarly, (NH4)3Fe(SO4)3 is first obtained, and then is decomposes into NH4Fe(SO4)2. CaSO4 forms from CaCO3 and PbO transformes into PbSO4 when reaction temperature is above 400 ℃.
Key words: low grade zinc oxide ore; ammonium sulfate; reaction process; phase transformation behavior
Foundation item: Project(51204054) supported by the National Natural Science Foundation of China; Project (2014CB643405) supported by the National Basic Research Program of China; Project(N150204009) supported by the Fundamental Research Funds for the Central Universities, China
Received date: 2015-11-23; Accepted date: 2016-05-20
Corresponding author: SHEN Xiao-yi; Tel: +86-24-83687731; E-mail: shenxy@smm.neu.edu.cn
(编辑 龙怀中)
基金项目:国家自然科学基金资助项目(51204054);国家重点基础研究发展计划项目(2014CB643405);中央高校基本科研业务费专项资金资助(N150204009)
收稿日期:2015-11-23;修订日期:2016-05-20
通信作者:申晓毅,副教授,博士;电话:024-83687731;E-mail: shenxy@smm.neu.edu.cn