Evolution of eutectic structures in Al-Zn-Mg-Cu alloys during heat treatment
来源期刊:中国有色金属学报(英文版)2006年第3期
论文作者:樊喜刚 蒋大鸣 孟庆昌 张宝友 王涛
文章页码:577 - 581
Key words:Al-Zn-Mg-Cu alloy; Al2CuMg phase; eutectic structure; heat treatment
Abstract: The evolution of the eutectic structures in the alloys with different copper contents during heat treatment was studied by scanning electron microscopy(SEM), energy dispersive X-ray spectroscopy(EDS), and differential scanning calorimetry(DSC). The as cast microstructures involve α(Al), eutectic(α(Al) + Mg(Al, Cu, Zn)2) and Al7Cu2Fe. The Al2CuMg particles form during heat treatment. The volume of coarse phases decreases quickly in the initial 12 h during heat treatment. The volume of coarse phases change a little at 400 and 420 ℃. Copper content has a great influence on the evolution of the eutectic. The coarse phases dissolve slowly in alloy with higher copper content.
基金信息:the Science and Technology Development Fund of Harbin, China

FAN Xi-gang(樊喜刚)1, JIANG Da-ming(蒋大鸣)1, MENG Qing-chang(孟庆昌)1,
ZHANG Bao-you(张宝友)1, WANG Tao(王 涛)2
1. Analysis and Measurement Center, Harbin Institute of Technology, Harbin 150001, China;
2. Northeast Light Alloy Co Ltd, Harbin 150060, China
Received 20 July 2005; accepted 14 December 2005
Abstract: The evolution of the eutectic structures in the alloys with different copper contents during heat treatment was studied by scanning electron microscopy(SEM), energy dispersive X-ray spectroscopy(EDS), and differential scanning calorimetry(DSC). The as cast microstructures involve α(Al), eutectic(α(Al) + Mg(Al, Cu, Zn)2) and Al7Cu2Fe. The Al2CuMg particles form during heat treatment. The volume of coarse phases decreases quickly in the initial 12 h during heat treatment. The volume of coarse phases change a little at 400 and 420 ℃. Copper content has a great influence on the evolution of the eutectic. The coarse phases dissolve slowly in alloy with higher copper content.
Key words: Al-Zn-Mg-Cu alloy; Al2CuMg phase; eutectic structure; heat treatment
1 Introduction
The solute redistribution during solidification leads to microsegregation and the formation of coarse intermetallic particles, which can significantly influence the properties and productivity of the 7000 series aluminium alloys[1]. In order to decrease the micro- segregation and dissolve the eutectic, homogenization is necessary for as cast alloy before subsequent processing. Some coarse constituents may exist in alloy after homogenization, including large eutectic structures and coarse particles formed during homogenization, which influence the formability, recrystallization behavior and the properties of the alloy. The coarse particles with size larger than 1 μm will deteriorate the properties, such as the toughness and fatigue performance[2-6]. In 7000 aluminium alloys, these detrimental particles are especially the iron rich and copper rich intermetallic phases.
Copper was added into the 7000 alloys to improve the stress corrosion cracking(SCC) resistance[7, 8]. More remnants of coarse copper rich phases might be presented with increasing copper content, which were detrimental to the toughness and degraded the age hardenability of the alloy[9-11].
The Al-Zn-Mg-Cu system was complex. It was reported that several intermetallic phases such as η(MgZn2), T(Al2Mg3Zn3), S(Al2CuMg), θ(Al2Cu) Al7Cu2Fe, Al13Fe4 and Mg2Si can occur below the solidus[12-14]. The η and T phases were often presented as solid solutions with extended composition ranges containing all four elements. The second phases based on η(MgZn2), T(Al2Mg3Zn3) and S(Al2CuMg) were present in the as cast Al-Zn-Mg-Cu alloys, and the nature of these phases was reported[15]. The stepped heat treatments on the dissolution of soluble remnant constituents were also investigated in the 7055 alloy[16]. Although homogenization and the nature of the coarse phases existing in the as cast and as homogenized Al-Zn-Mg-Cu alloy were investigated, the study of the transformation from the eutectic structures to coarse particles during homogenization was very limited in these alloys. It is timely to investigate the evolution of coarse phases in commercial alloys.
The objective of this study is to understand the influence of the copper content, temperature and time on the evolution of the coarse particles of Al-Zn-Mg-Cu alloys during solidification and heat treatment. The transformation from eutectic structures to coarse particles was also investigated.
2 Experimental
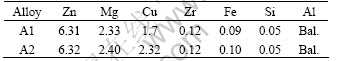
Two alloys were prepared in the range of commercial 7050 and 7010 alloys in compositions. The chemical compositions are given in Table 1.
Table 1 Chemical compositions of two alloys (mass fraction, %)
The ingots (200 mm in diameter) were produced by using semi-continuous casting method in the Northeast Light Alloy Co Ltd. Specimens for heat treatment were cut at 1/2 radius from the center of the ingot. To study the evolution of the microstructures in as cast alloys during heat treatment, the specimens were heated at 400, 420, 440 and 460 ℃, and held for different periods (6, 12 and 24 h) then quenched into the water. The heating temperature variation was controlled within ±5 ℃.
Optical microscopy was used to examine the size, shape and volume fraction of the coarse phases. Image analysis was used to measure the coarse phases. Scanning electron microscopy(SEM) was used to analyze the phases, along with energy dispersive spectroscopy to determine their chemical compositions. SEM work was conducted on an S-4700 SEM. DSC experiments were performed using a NETZSCH STA 449C calorimeter.
3 Results
3.1 Microstructures of as cast alloys
The as cast microstructures are shown in Fig.1. The dendrites are separated by the coarse phases. The white areas are α(Al) and the black areas are eutectic as reported in Ref.[12]. The two alloys exhibit similar as cast structures, the eutectic form an almost continuous network. The area fraction of the eutectics is nearly 5.7%.
3.2 Evolution of microstructures during heat treat- ment
The area fraction of the eutectics and the coarse phases in alloy A1 is close to alloy A2. The fraction of the eutectic gradually decreases during heat treatment, and the difference of area fraction between the two alloys was observed. The characterization of eutectic structures disappears gradually with heat treatment, and continuous mixture of phases evolves into isolate particles. After 24 h homogenization at 460 ℃, the fraction of coarse particles in alloy A1 is lower than that in alloy A2. The samples were quenched into the cold water after heat treatment, so there are no small particles precipitate in the grains.
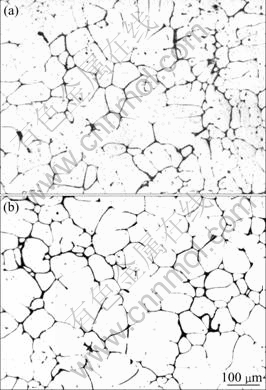
Fig.1 Optical micrographs of as cast alloys: (1) Alloy A1; (2) Alloy A2
The quantitative analysis results for the fraction of the phases other than the α dendrites are shown in Fig.2. From Fig.2(a), it can be seen that the particles fraction in alloy A1 decreases with increasing holding time at different temperatures. The fraction of coarse particles decreases sharply in the initial 6 h at 440 and 460 ℃, and changes little within further holding. There is small change of the fraction at 400 and 420 ℃ in alloy A2 as shown in Fig.2(b). The fraction of residual coarse particles in alloy A1 is lower than that in alloy A2 under the same heat treatment condition.
The evolution of the microstructures during heat treatment is investigated by using SEM. Fig.3(a) and Fig.3(b) show the lamellar as cast eutectic structure. The eutectic is believed as α-Al and MgZn2[12], which will be analyzed later. Some coarse particles (arrowed in Fig.3(b)) embedded into the eutectic structures were also observed. EDS analysis results(Table 2) show that the coarse particles contain more Cu and Fe. Its composition is close to stoichiometric Al7Cu2Fe phase. So the coarse particles can be identified as Al7Cu2Fe.
The eutectic disappeared after 24 h homogenization at 460 ℃. The dominant particles in alloy A1 are Al7Cu2Fe, as shown in Fig.3(c). In alloy A2, there are more Cu-rich particles (arrowed in Fig.3(d)) besides the Al7Cu2Fe particles. From Fig.3(d), it can be seen that the Al7Cu2Fe is brighter than the Cu-rich particles. The EDS result of the Cu-rich particles is shown in Table 2, which is close to Al2CuMg phase in composition. The higher fraction of coarse particles in alloy A2 is resulted from the more Cu-rich particles. The morphology of the Al7Cu2Fe particles is not regular, while the Cu-rich particles exhibit as elliptical shape (Fig.3(e)). Especially the serrated border of Al7Cu2Fe particles is observed in Fig.3(f).

Fig.2 Evolution of area fraction of phases other than α dendrites during heat treatments: (a) Alloy A1; (b) Alloy A2
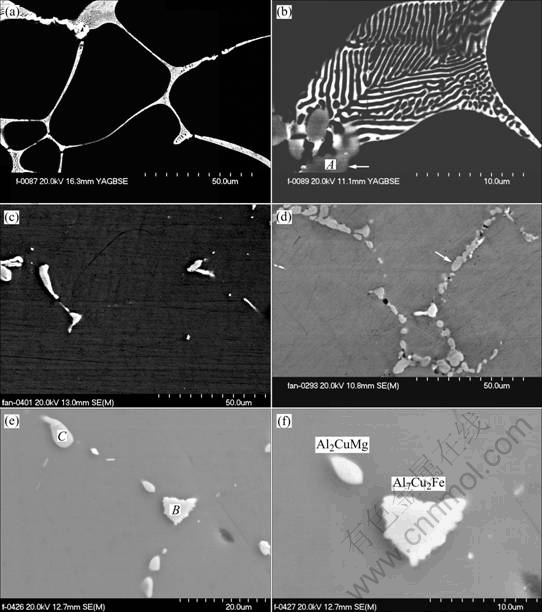
Fig.3 SEM micrographs of two alloys under different heat treatment conditions: (a) As cast microstructures; (b) High magnification of Fig.3(a); (c) Alloy A1(460 ℃, 24 h); (d) Alloy A2 (460 ℃, 24 h); (e) Morphology of coarse particles; (f) High magnification of Fig.3(e)
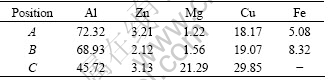
Table 2 Results of EDS analysis of various types of intermetallic phases shown in Fig.3(e) (mole fraction, %)
The differential scanning calorimetry(DSC) results are shown in Fig.4. Two sharp endothermic processes appear around 480 and 498℃. The endothermic peak 1 can be ascribed to the melting of the eutectic (α-Al+MgZn2). Peak 2 can be attributed to the melting of Al2CuMg[14]. There is only one endothermic peak in as cast alloy A2, indicating the existence of α-Al and MgZn2 in the as cast
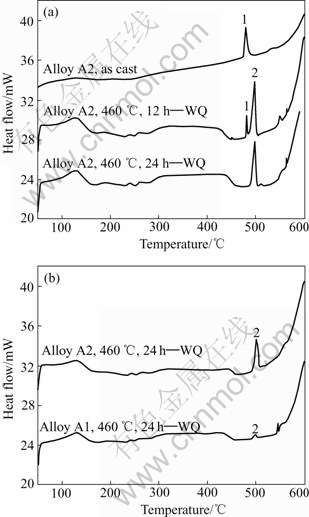
Fig.4 DSC curves under different heat treatment conditions: (a) Alloy A2 under different conditions; (b) For two alloys
structures. No peak associated with the Al2CuMg phase in as cast alloy was observed.
It should be noted that the weaker endothermic peak indicates the decrease of the fraction of the eutectic. Endothermic peak 2 corresponding to the melting of Al2CuMg appeared after 12h homogenization at 460 ℃, indicating the formation of Al2CuMg during homogenization. After 24 h homogenization at 460 ℃, the endothermic (peak 1) disappears as shown in Fig.4(a), which indicates that all the MgZn2 disappears. It is in agreement with the SEM analysis results(Figs.3(c) and 3(d)). Furthermore, the endothermic peak 2 in alloy A2 is stronger than that in alloy A1(Fig.4(b)), which indicates that more Al2CuMg particles exist in alloy A2.
4 Discussion
The microstrues of the two alloys reveal a mixture of dendritic α phase and lamellar eutectic formed between the dendrites. The two alloys exhibited similar as cast structures, which suggested that the different copper content had only a little influence on the solidification process. The DSC result showed that the eutectic contains α-Al and MgZn2. The EDS results show that the eutectic contain more copper(Table 3), which shows that some Zn lattices are substituted by Cu atom in MgZn2 phase. It is consistent with the report that Mg(Al, Cu, Zn)2 are solid solutions with extended composition ranges containing four elements[8].
Table 3 Results of EDS analysis of eutectic structures in as cast alloys (mole fraction, %)
During the heat treatment, the fraction of phases other than the α dendrites decreases, and the decreasing rate in alloy A1 is higher than that in alloys A2. DSC and SEM results demonstrate that the coarse Al2CuMg particles form. More Al2CuMg particles exist in alloy A2 under the same condition. This suggests that the copper content has a great influence on the evolution of eutectic structures during the heat treatment.
In the as cast alloys, there will be some segregation regions with more Cu, Zn and Mg. The segregation of solute occurring during solidification leads to more Cu dissolving in the eutectic. It is difficult to eliminate the segregation within the grains after the heat treatment. The Cu content in the eutectic in alloy A2 is higher than that in alloy A1 as listed in Table 3. The copper has lower diffusion coefficient as compared with zinc and magnesium[17]. The higher Cu content in the eutectics in alloy A2 slows down the diffusion process during heat treatment. Furthermore, the high supersaturation extent of Cu in alloy A2 will provide more Cu solutes to form Al2CuMg and lead to more Al2CuMg particles. Hence, the alloy with higher Cu content contained more Al2CuMg particles under the same heat treatment condition.
The eutectic disappeared after 24 h homogenization at 460 ℃. There are some coarse intermetallic phases remain in the grain boundaries. These particles are identified as either insoluble phase (the iron rich itermetallic Al7Cu2Fe), or Al2CuMg phase, which is in agreement with the results made by ROBSON[2]. The dominant particles remained in alloy A1 are Al7Cu2Fe, whereas Al2CuMg in alloy A2. The practical homogenization temper is about 460 ℃, 24-36 h for 7000 series alloy, which can dissolve the coarse particles to a great extent in alloy A1. But, for alloy A2, further study about homogenization should be made to reduce the fraction of coarse intermetallic phases, and optimize the processing practices.
5 Conclusions
1) The two as-cast alloys exhibited similar structures, containing α(Al), the eutectic(α(Al)+ Mg(Al, Cu, Zn)2) and Al7Cu2Fe phases.
2) The lamellar eutectic dissolved into the matrix or evolved into isolated particles during the heat treatment. The particles present in the alloys after heat treatment for 24 h at 460 ℃ are Al7Cu2Fe or Al2CuMg.
3) The fraction of the phases other than the α dendrite decreases with the increasing of holding time. The fraction of these phases in the alloy with higher copper content is higher during the heat treatment. The excessive phase in alloy with higher copper content is Al2CuMg.
4) Copper content has a great influence on the dissolution of eutectic structures. The Al2CuMg phase formed during the heat treatment.
Acknowledgements
The financial support of the Science and Technology Development Fund of Harbin is greatly acknowledged. The authors are also grateful to the Northeast Light Alloy Co Ltd for production of materials.
References
[1] Totik Y, Gavgali M. The effect of homogenization treatment on the hot workability between the surface and the center of AA 2014 ingots [J]. Materials Characterization, 2003, 49(3): 261-268.
[2] Robson J D. Microstructural evolution in aluminium alloy 7050 during processing [J]. Mate Sci Eng A, 2004, 382:112-121.
[3] Gurbuz R, Alpay S P. The effect of coarse second phase particles on fatigue crack propagation [J]. Scripta Metallurgica et Materialia, 1994, 30(11): 1373-1376.
[4] CHEN Kang-hua, LIU Yun-zhong, LIU Hong-wei. Microstructure and mechanical properties of enhanced solution treated 7075 and 2024 aluminium alloys [J]. The Chinese Journal of Nonferrous Metals, 2000, 10(6): 819-822.(in Chinese)
[5] XIE F Y, KRAFT T, ZUO Y, MOON C H, CHANG Y A. Microstructure and microsegregation in Al-rich Al-Cu-Mg alloys [J]. Acta Materialia, 1999, 47(2): 489-500.
[6] Joseph H M, Hyman R. Influence of nonequilibrium second-phase formed during solidification upon the mechanical behavior of an aluminium alloy [J]. Metall Trans, 1971, 2(2): 427- 432.
[7] Sarkar B, Marek M, Starke E A Jr. Effect of copper content and heat treatment on the stress corrosion characteristics of Al-6Zn-2Mg-XCu alloys [J]. Metall Trans A, 1981, 12(11): 1939-1943.
[8] Deschamps A, Bréchet Y, Livet F. Influence of copper addition on precipitation kinetics and hardening in Al-Zn-Mg alloy [J]. Mate Sci Tech, 1999, 15: 993-1000.
[9] Thompson D S. Metallurgical factors affecting high strength aluminum alloy production [J]. Metall Trans A, 1975, 6(4): 671-683.
[10] Vasudevan A K, Doherty R D. Grain boundary ductile fracture in precipitation hardened aluminium alloys [J]. Acta Metallurgy, 1987, 35(6): 1173-1219.
[11] Dumont D, Deschamps A, Bréchet Y. On the relationship between microstructure, strength and toughness in AA7050 aluminum alloy [J]. Mate Sci Eng A, 2003, 356: 326-336.
[12] Xie F Y, Yan X Y, Ding L, Zhang F, Chen S L, Chu M G, Chang Y A. A study of microstructure and microsegregation of aluminum 7050 alloy [J]. Mate Sci Eng A, 2003, 335: 144-153.
[13] Rokhlin L L, Dobatkina T V, Bochvar N R, Lysova E V. Investigation of phase equilibria in alloys of Al-Zn-Mg-Cu-Zr-Sc system [J]. Journal of Alloys and Compounds, 2004, 367: 10-16.
[14] Li X M, Starink M J. Effect of compositional variations on characteristics of coarse intermetallic particles in overaged 7000 aluminium alloys [J]. Mate Sci Tech, 2001, 17: 1324-1328.
[15] Mondal Chandan, Mukhopadhyay A K. On the nature of T(Al2Mg3Zn3) and S(Al2CuMg) phase present in as-cast and annealed 7055 aluminum alloy [J]. Mate Sci Eng A, 2005, A391: 367-376.
[16] Chen K H, Liu H W, Zhang Z, Li S. Todd Richard I. The improvement of constituent dissolution and mechanical properties of 7055 aluminum alloy by stepped heat treatments [J]. Journal of Materials Processing Technology, 2003, 142: 190-196.
[17] LIU X T, Dong J, Cui J Z, Zhao G. Homogenizing treatment of high strength aluminium alloy cast under electric magnetic field [J]. The Chinese Journal of Nonferrous Metals, 2003, 13(4): 909-913.(in Chinese)
Foundation item: Project(2004AA5BG018) supported by the Science and Technology Development Fund of Harbin, China
Corresponding author: FAN Xi-gang; Tel: +86-451-86417617; E-mail: xigangfan@163.com

