Trans. Nonferrous Met. Soc. China 20(2010) s826-s831

Morphological evolution of non-dendritic microstructure during solidification under stirring
LIN Xin(林 鑫), TONG Lei-lei(统雷雷), ZHAO Li-ning(赵力宁),
WANG Li-lin(王理林), WANG Meng(王 猛), HUANG Wei-dong(黄卫东)
State Key Laboratory of Solidification Processing, Northwestern Polytechnical University, Xi’an 710072, China
Received 15 March 2010; accepted 25 June 2010
Abstract:Morphological evolution of non-dendritic microstructure during the solidification of succinonitrile-5%water (SCN-5%Wat) transparent alloy under mechanical stirring was experimentally investigated. The incubation time for the formation of non-dendritic microstructures decreased rapidly and the microstructure was gradually refined with the increase of stirring rate and cooling rate. When the stirring rate and cooling rate were low, the incubation time for the formation of non-dendritic microstructures decreased obviously with the increase of the melt undercooling. When the stirring rate was high, the effect of the melt undercooling on the incubation time for the formation of non-dendritic microstructures was weak. The morphology of primary microstructures had an important influence on the subsequent morphological evolution of these microstructures during the isothermal holding of the melt without stirring. It was found that when there were dendrites in the undercooled melt, the dendrites would be dissolved and the final microstructure would be replaced by the re-nucleated globular crystals if the stirring time was long enough.
Key words: non-dendritic; globular crystal; morphological evolution; stirring; cooling rate
1 Introduction
With the development of a new near net shaping technology—semi-solid processing technology, the formation mechanism of semi-solid globular crystal attracts more and more researchers’ attention. During the semi-solid processing, the formation of non-dendritic or globular crystal microstructure with the appropriate size through shearing can improve the fluidity and uniformity of materials and eliminate the defects, such as solidification segregation, shrinkage porosity and shrinkage cavity, which thereby improves the comprehensive mechanical properties of the materials. At present, most of understandings on the formation mechanism of the semi-solid globular crystal are still based on the research on the microstructural behavior characteristics of the semi-solid metal in the early 1970s by Flemings et al[1-2], namely, reckon that the formation of semi-solid non-dendritic or globular microstructure results from the fragmentation of the dendrites. Molenaar et al[3] proposed that the stirring decreases the solute enrichment ahead of the solid/liquid interface, so that a small temperature gradient will lead the crystal to grow in cellular pattern and form the non-dendritic or rosette-type microstructure. However, Vogel et al[4] thought that the crystal would grow dendritically in the initial solidification and the dendrite arm generally could not be fractured directly but bent during the stirring due to its high plasticity and low strength under high temperature. Hellawell[5] also thought that the dendrite arms could not be fractured directly by the melt shearing, but bent elastically or plastically. However, he thought that the remelting of the dendrite arms caused by the temperature perturbation under flow is the main reason for the formation of the globular microstructure during semi-solid solidification process. In recent years, more and more researchers [6-11] thought that the globular microstructure during semi-solid solidification process forms from the natural nucleation and their subsequent crystal growth in the melt under stirring. However, most of the present research mainly focus on the nucleation behavior of globular crystals in the undercooling melt under stirring while the solid-liquid interface morphology stability during the subsequent globular crystal growth process is lack of adequate analysis. In the present work, the transparent model alloy experiments are carefully designed for in-situ observation on semi-solid non-dendritic morphological evolution, and the effects of melt undercooling and cooling rate on non-dendritic morphological evolution were investigated.
2 Experimental
Succinonitrile-5%water alloys(mole fraction) were prepared from 99.99% succinonitrile and distilled water. The specimen cell was kept sealed to ensure the same alloy composition throughout the entire experiment. According to SCN-Wat phase diagram[12], SCN-5%Wat alloy has the liquidus temperature of 49.9 ?C, the monotectic temperature of 18.8 ?C, and the freezing temperature range of 31.1 ?C. Thus, the experimental temperature range was controlled in 46-48?C, corresponding to the melt undercooling of 1.9-3.9 ?C. A self-made apparatus was designed for in situ observation of the transparent semi-solid alloy, as shown in Fig.1. The diameter of central cylinder cavity was 3 mm, and the spacing between the upper and lower glass was 3 mm. The temperature of SCN-5%Wat alloy melt was controlled using a constant temperature water bath, whose temperature control accuracy is better than 0.05 ?C. The rotation of the outer cylinder of the apparatus driven by a magnetic device brought a shearing of the alloy melt. In situ observation was performed using a stereomicroscope and JVC video camera.
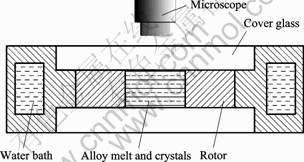
Fig.1 Schematic of experimental apparatus
3 Results and discussion
3.1 Effects of cooling rate and melt undercooling on incubation time for formation of non-dendritic microstructures
The melt cooling rate and undercooling have an important influence on the morphology of solidification microstructures. In the previous study on semi-solid processing[13], it was found that the particle size can be reduced significantly with the increase of cooling rate. In the present experiment, SCN-5%Wat alloy was first heated to 55 ?C and sheared isothermally at the stirring rate of 500 r/min for 30 min to eliminate non-uniform distribution of solute from solidification segregation. Then, the melt was cooled at a certain cooling rate without stirring. When the melt temperature reached a set temperature, the stirring was restarted at a set shearing rate and the formation of globular crystals was observed. The results are shown in Table 1. The incubation time was defined as the time from the beginning of the stirring to emergence of non-dendritic microstructure, because the crystal growth in the undercooled stirring melt was very quick after nucleating. It was shown that, the incubation time decreased rapidly with the increase of the cooling rate. When the cooling rate was low, the incubation time for the formation of globular crystals decreased obviously with the increase of the melt undercooling. When the cooling rate was high, the effect of the melt undercooling on the incubation time was weak.
Table 1 Incubation time for formation of non-dendritic microstructure with different stirring rate and cooling rate
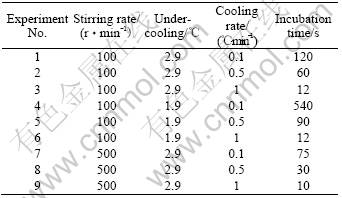
Fig.2 shows the rosette-type and globular microstructure morphology in SCN-5%Wat alloy melt which was cooled to a melt undercooling of 2.9 ?C under three different cooling rates vc of 0.1, 0.5 and 1 ?C/min, respectively, then sheared with two different stirring rates of 100 and 500 r/min, respectively. It can be seen that, the microstructures under vc=1 ?C/min was significantly refined, compared with the microstructures under vc=0.1 ?C/min and vc=0.5 ?C /min. This is due to the fact that the high cooling rate can increase the heterogeneous nucleation rate in the alloy melt and produce more primary non-dendritic microstructure, meanwhile, the high cooling rate also reduce the time to reach the set melt temperatures, which had contributed to the smaller globular microstructure. This is consistent with the observation of the morphological evolution of primary crystal in semi-solid solidification of Zn-Al alloy by ZHang[14].

Fig.2 Influence of cooling rate and stirring rate on primary microstructure in SCN-5%Wat alloy melt under undercooling of 2.9?C:
(a) Stirring for 120 s at 100 r/min, vc=0.1?C /min; (b) Stirring for 60 s at 100 r/min, vc=0.5?C /min; (c) Stirring for 12 s at 100 r/min,
vc=1?C /min; (d) Stirring for 75 s at 500 r/min, vc =0.1?C /min; (e) Stirring for 30 s at 500 r/min, vc=0.5?C /min; (f) Stirring for 10 s at 500 r/min, vc=1 ?C /min
In addition, it was found that the globular microstructures formed at a high stirring rate, while the rosette-type microstructures formed at a low stirring rate. Schulze et al[15] found that a time-periodic lateral flow, with the appropriate selection of the frequency and amplitude of the modulation, both physically realizable, can eliminate the possibility of morphological instability for a significant range of solute concentrations in the directional solidification. Define e=U/V, which measures the amplitude U of the lateral oscillations of fluid velocity in units of interface velocity V, supposing the lateral direction is perpendicular to the interface moving direction. They found that the interface stability increased with the increase of e. If any local part on the globular crystal interface was treated as flat interface, the stability analysis of Schulze and Davis[15] on directional solidification can be used as a good approximate analysis for semisolid solidification. The crystal rotation, driven by the melt shearing under the stirring, could be explained as that the lateral flow with proper frequency and amplitude parallel to the local liquid-solid interface of the crystals existed[7]. At high stirring speed, the crystals could be stable and grow globularly because the melt flow was turbulent, namely, e was large; at low stirring speed, the crystals was easy to destabilize and formed rosette-type growth because the melt flow was relatively stable, namely, e was small.
3.2 Morphological evolution of non-dendritic microstructure
The evolutions of rosette-type microstructures and globular microstructures under the stirring and the isothermal holding were further investigated. The alloy was first heated to 55 ?C and held for 30 min under the stirring at 500 r/min to eliminate non-uniform distribution of solute. Then, the alloy melt was cooled to 48 ?C (the undercooling of 1.9 ?C) at the cooling rate of 0.1 ?C /min without stirring. For investigating the evolution of rosette type microstructures, the continued stirring was restarted at 100 r/min, and two cases were studied: 1) the stirring was stopped immediately when a large number of rosette-type microstructures emerged; 2) the stirring was still kept when a large number of rosette-type microstructures emerged. For investigating the evolution of globular microstructures, the continued stirring was restarted at 500 r/min, and the stirring was stopped immediately when a large number of globular microstructures emerged. Then, the evolution of microstructures under the isothermal holding was investigated.
For the evolution of rosette-type microstructures, it is found that, when the rosette-type microstructures were first formed (Fig.3(a)), if the stirring was stopped, they will transform into dendritic microstructures after 5 min (Fig.3(b)), while, if the stirring was kept, the rosette-type growth was continued, meanwhile, the globular particles increased apparently, as shown in Fig.3(c).
For the evolution of globular microstructures, it is found that, when the globular microstructures first emerged at a high stirring rate (Fig.3(d)), if we stopped stirring, the globular crystals will continuously grow till their size reached a certain value (Fig.3(e)). Meanwhile, the large globular crystals began to merge small ones to become larger when they contact each other (Fig.3(f)), which is driven by interfacial energy. On comparison among the above results, it can be seen that the stirring was beneficial to the stable growth of non-dendritic microstructures. From comparing Fig.3(b) with Fig.3(e), it can also be found that the initial solid-liquid interface morphology has an important influence on the stability of the crystal during the subsequent interface development.
Li[16] proposed a coefficient S corresponding to the relative stability of the interface:
 (1)
(1)
where
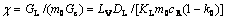
W=2Pc(1-k0) - (l+1)
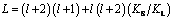
where l is the order of spherical harmonics, cR is the solute concentration at the undisturbed spherical interface, k0 is solute partition coefficient, KL and KS are the thermal conductivities in the liquid and solid respectively, LV is the latent heat of melting, DL is the solute diffusion coefficient in the liquid, GL and GC are the temperature gradient and. concentration gradient at the undisturbed spherical interface in the liquid respectively, m0 is the liquidus slope, Pc=VR/(2DL), where Pc is the Peclec number, V is the globular crystal growth rate, R is the globular crystal radius, c is a parameter evaluating the relative size between the temperature gradient GL and the concentration gradient GC.
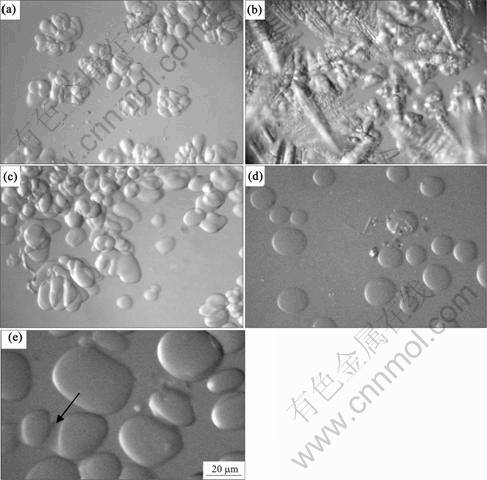
Fig.3 Morphological evolution of non-dendrite microstructure under undercooling of 1.9 ?C:(a) Stirring for 5 400 s at 100 r/min; (b) After stopping stirring for 5 min; (c) Continue to stir at 100 r/rmin for 5 min; (d) Stirring for 275 s at 500 r/min; (e) After stopping stirring for 60 min
For the alloys, the stability of the globular crystal increases if S>1. As Pc is constant, the value of S is determined by c, which depends on solute concentration cR in the liquid at undisturbed spherical interface. As the interfacial liquid concentration decreases, the value of c increases. When c increases to a certain extent, which leads S to be greater than 1, denoting that the relative stability of the globular crystal increases, the globular crystal can grow in approximate spherical morphology to a certain size, which is larger than the critical nucleation radius; on the contrary, as the interfacial concentration increases, the relative stability of the globular crystal decreases and the globular crystal will destabilize and grow in the dendritic morphology. The stirring enhances the effect of far-field melt with the low solute concentration on the interface, which is beneficial to decreasing the liquid concentration at the solid-liquid interface. Because the stirring rate for the initial formation of the globular crystals is much higher than that for the initial formation of rosette-type microstructures, it can be reasonable that the liquid concentration at the solid-liquid interface of globular crystals is lower than that of rosette-type microstructures when the stirring was stopped. Thus, the relative stability of the globular crystals is high and the globular crystals could continue to maintain the stable growth under the following isothermal holding process without stirring.
From Fig.3(e), it can be seen that, the globular crystals could grow to the size of more than 100 μm, which is much higher than the critical stability radius for the globular crystal growth under the pure solute diffusion condition. It is believable that it is greatly related to solid fraction at that time. When the crystal size is very small and the solid fraction is low, the interaction among the solid particles is very weak. Therefore, as an approximate isolated crystal, the globular crystals will become unstable and grow dendritically when the globular crystals reach a critical stability radius. However, when the crystal size is large and the solid fraction is high, the overall average concentration of the undercooled melt would increase since the solute partition coefficient is less than 1, which leads the subsequent growth of the crystal to be influenced remarkably by its neighboring crystals. In this case, the crystal growth is different from the growth of an isolated crystal. As the liquid fraction is relatively small, the diffusion distance of the discharged solute from the solid-liquid interface is shortened, so the liquid concentration around the crystals begins to increase gradually from the initial concentration of the melt, which results in the decrease of the melt undercooling and the decrease of the growth driving force for the globular crystals. The globular crystals stop growing when the growth driving force disappears. This is the reason that the semi-solid globular crystals stop growing when a certain solid fraction is reached as observed in Fig.3. The melt temperature could be still below its liquidus temperature at this time, however, the melt undercooling has become very small, which leads growth driving force to become weak gradually, and the crystals would not grow dendritically, and the merging and coarsening among the crystals become dominant.
3.3 Morphological evolution of dendritic microstructure under stirring
In order to further investigate the morphological evolution of the dendrites under the stirring, the alloy was first heated to 55 ?C and held for 30 min under the stirring at 500 r/min to realize the homogenization treatment. Then, the alloy melt was cooled to 46.3 ?C(the undercooling of 3.6 ?C) at the cooling rate of 0.1?C/min without stirring. Then, the stirring was restarted at 1 000 r/min to perform the isothermal melt shearing. It is found that the columnar dendrites with well-developed secondary dendritic arms grew inside from the cylinder wall when the melt was undercooled at 46.3 ?C, as shown in Fig.4(a). When the isothermal melt stirring at 1 000 r/min began, the dendritic arms were dissolved and became passive gradually and the globular crystals nucleated and grew up gradually in the melt. A coexistence of the globular crystals and the dendrites can be found as shown in Fig.4(b). After the stirring for 60 min, the dendritic arms were completely dissolved, and the final microstructures consisted of the globular crystals completely as shown in Fig.4(c).
For the typical dendritic growth in undercooled melt, there is a solute boundary layer at the dendritic arm tip, in which the solute concentration gradient is negative. The greater the absolute value of the solute concentration gradient is, the greater the growth velocity of dendrite is. For the absence of the forced convection, the solute concentration gradient at dendritic arm tip is greater than that of the sides of the dendritic arms, so the growth velocity of dendrite arm tip is greater than that of the sides of the dendritic arms. With the introduction of the forced convection, the concentration gradient in the solute boundary layer is homogenized due to the effect of the shear flow around the dendrites, and the growth velocities at the different parts in the dendrite tend to be the same. This could be the reason of the dendritic passivation. Besides, the dissolving of the dendritic arms could be attributed to the possible local temperature increase of the alloy melt caused by the mechanical work of the stirring.
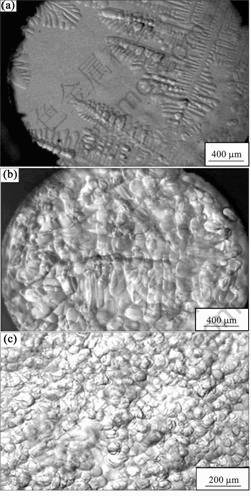
Fig.4 Morphological evolution of dendritic microstructure under isothermal stirring under undercooling of 3.6 ?C: (a) Columnar dendritic growth; (b) Coexistence of globular crystals and dendrites; (c) Globular microstructure
4 Conclusions
1) The incubation time for the formation of non-dendritic microstructures decreased rapidly and the microstructure was gradually refined with the increase of the stirring rate and cooling rate. When the cooling rate was low, the incubation time for the formation of non-dendritic microstructures decreased obviously with the increase of the melt undercooling. When the cooling rate was high, the effect of the melt undercooling on the incubation time for the formation of non-dendritic microstructures was weak. The globular microstructure formed at high stirring rate, while the rosette-type microstructure formed at low stirring rate.
2) The stirring could increase the interface relative stability of the non-dendritic microstructure. The morphology of primary microstructure had an important influence on the subsequent development of microstructure during the isothermal holding process. The primary rosette-type microstructure will be destabilized to well-developed dendritic microstructure in a short time during the isothermal holding process, while the primary globular microstructure could keep the globular growth to a larger size.
3) The globular microstructure could form through the direct nucleation in the stirring melt. Even when there were dendrites in the undercooled melt, the dendrites would be dissolved and the final microstructure would consist of the globular crystals completely if the stirring time was long enough.
References
[1] FLEMINGS M C. Behavior of metal alloys in the semisolid state [J]. Metallurgical Transactions A,1991, 22: 957-981.
[2] SPENCER D B, MEHRABIAN R, FLEMINGS M C. Rheological behaviour of Sn-15Pct Pb in the crystallization range [J]. Metallurgical Transactions A,1972, 3: 1925-1932.
[3] MOLENAAR J M M, KATGERMAN L, KOOL W H, SMEULDERS R J. On the formation of the stircast structure [J]. Journal of Materials Science,1986, 21: 389-394.
[4] VOGEL A, CANTOR B. Stability of a spherical particle growing from a stirred melt [J]. Journal of Crystal Growth, 1977, 37: 309-316.
[5] HELLAWELL A. Grain evolution in conventional and rheo-castings [C]//Proceedings of the 4th International Conference on Semi-Solid Processing of Alloys and Composites. Sheffield, 1996: 60-65.
[6] FAN Z. Semisolid metal processing [J]. International Materials Review, 2002, 47: 49-85.
[7] LI T, LIN X, HUANG W D. Morphological evolution during solidification under stirring [J]. Acta Mater, 2006, 54: 4815-4824.
[8] LIN X, LI T, HUANG W D. Influence of rapid shear-rate changes on microstructure formation during semi-solid processing [J]. Solid State Phenomena, 2006, 116/117: 155-158.
[9] LI T, HUANG W D, LIN X. Formation of globular structure during semi-solid material processing [J]. The Chinese Journal of Nonferrous Metals, 2000, 10: 635-639. (in Chinese)
[10] JI S, FAN Z, BEVIS M J. Semi-solid processing of engineering alloys by a twin-screw rheomoulding process [J]. Materials Science and Engineering A, 2001, 299: 210-217.
[11] MARTINEZ R A, FLEMINGS M C. Evolution of particle morphology in semisolid processing [J]. Metallurgical and Materials Transactions A, 2005, 36: 2205-2210.
[12] JAMES E S J, DONALD O F, WILLIAM F K. A redetermination of the succinonitrile-water phase diagram [J]. Scripta Metallurgica, 1984, 18: 677-682.
[13] WU Q. The rheological behavior, microstructure and mechanical performance characteristics of aluminum-copper alloy [D]. Nanjing: Southeast University, 1990. (in Chinese)
[14] ZHANG S J. Stir casting characteristics, as-cast microstructure and tensile properties of Zn-Al alloy [D]. Nanjing: Southeast University,1993.(in Chinese)
[15] SCHULZE T P, DAVIS S H. Shear stabilization of morphological instability during directional solidification [J]. Journal of Crystal Growth, 1995, 149: 253-265.
[16] LI Tao. Researches on microstructure formation and rheology during semi solid processing through in situ observation technique [D]. Xi’an: Northwestern Polytechnical University, 2004. (in Chinese)
[17] FAHIEN R W. Fundamentals of transport phenomena[M]. New York: McGraw-Hill, 1983.
[18] WELTY J R, WICKS C E, WILSON R E. Fundamentals of momentum, heat and mass teansfer [M]. New York: Wiley, 1976.
(Edited by YUAN Sai-qian)
Foundation item: Project(50771083) supported by the National Natural Science Foundation of China; Project(02-TZ-2008) supported by State Key Laboratory of Solidification Processing in NWPU, China
Corresponding author: LIN Xin; Tel: +86-29-88494001; E-mail: xlin@nwpu.edu.cn