Effect of aggressive pH media on peat treated by cement and sodium silicate grout
来源期刊:中南大学学报(英文版)2011年第3期
论文作者:S. Kazemian A. Prasad B. B. K. Huat J. Bolouri Bazaz T. A. Mohammed F. N. Abdul Aziz
文章页码:840 - 847
Key words:peat; aggressive pH media; cementation; sodium silicate; grout; microstructure
Abstract: The effects of aggressive peat nature (pH) on the strength of peat treated by cement and cement-sodium silicate grout were investigated by evaluating the changes in unconfined compressive strength, moisture content, and scanning electron microscopy (SEM) of samples with time in different pH media. The results indicate that peats treated by cement-silicate have higher strength than peats treated by cement, due to an increase in pH value of the media. Furthermore, cement and cement-silicate are highly effective in reducing the moisture content and void ratio of the treated peats. The microstructures of treated peats support the laboratory test results.
J. Cent. South Univ. Technol. (2011) 18: 840-847
DOI: 10.1007/s11771-011-0771-x![]()
S. Kazemian1, A. Prasad2, B. B. K. Huat3, J. Bolouri Bazaz4,
T. A. Mohammed3, F. N. Abdul Aziz3
1. Department of Civil Engineering, Bojnourd Branch, Islamic Azad University, Bojnourd, Iran;
2. Department of Civil Engineering, Banaras Hindu University, Varanasi, India;
3. Department of Civil Engineering, University Putra Malaysia, Serdang, Malaysia;
4. Department of Civil Engineering, Ferdowsi University, Mashhad, Iran
? Central South University Press and Springer-Verlag Berlin Heidelberg 2011
Abstract: The effects of aggressive peat nature (pH) on the strength of peat treated by cement and cement-sodium silicate grout were investigated by evaluating the changes in unconfined compressive strength, moisture content, and scanning electron microscopy (SEM) of samples with time in different pH media. The results indicate that peats treated by cement-silicate have higher strength than peats treated by cement, due to an increase in pH value of the media. Furthermore, cement and cement-silicate are highly effective in reducing the moisture content and void ratio of the treated peats. The microstructures of treated peats support the laboratory test results.
Key words: peat; aggressive pH media; cementation; sodium silicate; grout; microstructure
1 Introduction
Peat is a soil with organic content of more than 75% [1], and ten degrees of humification (H1-H10) for its classification have been recommended by von POST [2] based on the botanical composition, degree of humification, and color of squeezed peat water. According to ASTM [3], the peat classification has been narrowed to three classes: fibrous, hemic and sapric. The fibrous peat (Fig.1(a)) is a peat with high organic and fiber contents and low degree of humification, and extremely acidic. The sapric peat (Fig.1(b)) is the most decomposed peat material. As compared with the firous peat deposits, the sapric peat deposits are likely to exist at lower void ratios and display lower permeability anisotropy, lower compressibility, lower friction angle, and higher coefficient of earth pressure at rest [4]. The behavior of the hemic peat (Fig.1(c)), in terms of the compressibility, shear strength and permeability, can be said to be intermediate between the fibrous and sapric peats.
The main specification of peat, impeding the cementing process, is acidic medium with a high ground water level [5-6]. The range of acidity level in peat nature is very wide. The pH of most peats is 2-6; but in some conditions where there is infiltration of brackish water, it can be as high as 7.8; if there is the presence of pyritic materials, it can be even less than 2 [7].
Deep stabilization (formation of cement columns) is proven to be an economical method to treat deposits of peat. However, the organic matter in peat is known to impede the cementing process in the soil, thus retarding the early strength gain of stabilized peat [8-12]. Increasing the number of solid particles filler such as fine sand or clay (kaolinite) may be added in soil stabilization to save cost. The filler itself may react but increase the strength of the soil by acting as a stiffener, particularly in peat, as these soils have extremely poor skeleton and often require large quantities of stabilizers [13]. AXELSSON et al [13] have carried out comprehensive trials and field works in which a portion of the cement, and cement with different industrial binders have shown positive results in the stabilization of organic soils.
The use of grouting and chemical grouting in geotechnical engineering applications has been expanded greatly in recent decades. Further, the last three decades have witnessed the introduction and use of many materials totally unrelated to the silicates. Today, commercial chemical grouts cover a wide range of materials, properties and costs, and give the grouter the opportunity to select a grout for specific job requirements. Sodium silicate is the basis for many chemical grout formulations in addition to the two shot injection of silicate-chloride. It is considered free of health hazards and environmental effects [14], and offers specific advantages for soil stabilization: 1) reliable and proven performance, 2) safety and ease of use, 3) environmental acceptability and compatibility, and 4) adaptability over a wide range of applications [15-16].

Fig.1 SEM images of peats: (a) Fibrous; (b) Hemic; (c) Sapric
Sodium silicates have been developed into a variety of different grout systems. These systems consist of sodium silicate and reactor/accelerator (like calcium chloride) which can be compatible with cement to get strong bonding properties in two-compound system, and sodium silicate and the reactant solution with cement can be injected separately in two steps. Two-compound system has been used in grouting below a water table and produces a high-strength, permanent grout if not allowed to dry out [17-18]. The ratios which are commonly used for sodium silicate grout system for soils have been reported by CIRIA [19].
KAROL [15] and USACE [18] explained that when sodium silicate solution and an appropriate solution of alkali metals salt (sodium and potassium) are mixed, the reaction forming a gel is virtually instantaneous. If the silicate solution has not been moved away (by groundwater or gravitational forces), it is penetrated by thin figers and lenses of chloride solution. Because the reaction is so rapid, not all the solutions can reach contact, but the unstable interface generally ensures that sufficient contact occurs to provide a continuous gel network through stabilized soil.
The testing program described herein was aimed at determining the effects of aggressive media on cementation and chemical reaction process of peats (fibrous, hemic, and sapric) treated by ordinary Portland cement (herein after called cement) and cement-sodium silicate grouts and kaolinite as filler (herein after called cement-silicate), and characterized by unconfined compressive strength (UCS), moisture content, and scanning electron microscopy (SEM).
2 Experimental
2.1 Materials
Peat was collected from various locations at Kampung Jawa, Kuala Lumpur in Malaysia to have all the three varieties: fibrous, hemic and sapric. The physico-chemical properties of fibrous, hemic and sapric peats were evaluated and are presented in Table 1. The degree of humification, cation exchange capacity (CEC) and surface area were evaluated in accordance with VON POST [3], GILLMAN and SUMPTER [21], and BET [22], respectively.
Table 1 Physico-chemical characteristics of peat

The BET method is based on measuring the quantity of adsorbate gas adsorbed on a solid surface by sensing the change in thermal conductivity of a flowing mixture of adsorbate and an inert carrier gas. The adsorbate was nitrogen and the inert carrier gas was helium. A small soil sample was placed in a sample tube and inserted into the cell holder. A Dewar flask was filled with liquid nitrogen and raised until the liquid nitrogen was close to the top of the cell. The flow of gas was started and nitrogen was adsorbed. The liquid nitrogen was removed and the desorption of the gas from the soil began. When the desorption was complete, the integrator displayed a number which was the sample surface area in square meters. Being divided by the sample mass gives the external specific surface area of the sample. This procedure was repeated two times and the average values are reported in Table 1. The SEM images of fibrous, hemic and sapric peats are shown in Fig.1.
Cement was sourced from Anuza Enterprise Company, Malaysia. The CaO and SiO2 contents were 65% and 21%, respectively, and the loss on ignition was 0.9%. Hydrous sodium silicate (syrupy liquid), which had less alkaline than metasilicates, was used as the second binding agent. The SiO2 content in sodium silicate was 28.7%, the silica ratio (SiO2/Na2O) was 3.22, and the pH value was equal to 11.3. Calcium chloride anhydrous powder (CaCl2) was used as reactor/ accelerator, with a minimum assay content equal to 96%.
The maximum impurities were free alkalinity [Ca(OH)2] 0.04%, sulphate (SO4) 0.02%, magnesium and alkalies (sulphate) 0.6%. Finally, kaolininit was added as filler and its main composition was SiO2 (45.8%) and Al2O3 (39.6%). Sodium silicate and calcium chloride were obtained from Merck Sdn. Bhd., Malaysia, and kaolinite was obtained from Bendosen Company, Malaysia.
2.2 Methods
The impact of different pH media on cementation chemical reactions was investigated by curing the treated peat samples in media having pH equal to 3, 5, and 7. Different pH media (3 and 5) were prepared by mixing appropriate amounts of HCl and NaOH in distilled water and the pH value was measured by dipping a pH probe into the solution at several times. In preparing the laboratory peat treated with cement and cement-silicate, the peats were first thoroughly homogenized with their natural water content by household mixer for 5 min and then cement (250 kg/m3). According to AXELSSON et al [13] for cement samples and based on CIRIA [19] recommendation for cement-silicate mixture, cement- silicate samples and kaolinite as filler were added separately and mixed into the peats. The stabilized peat samples were placed in the mold (50 mm in inside diameter and 120 mm in height) and compacted by hand. The prepared samples were extruded and soaked for curing up to 180 d.
The effectiveness of the treatment was evaluated by measuring the shear strength by performing UCS test (BS 1377: Part 7: 1990, Clause 7)[20] after curing for 45, 90 and 180 d. Further, to understand the microscopic structure of peats and the effect of cement and silicate on their properties, SEM was performed on some selected samples.
3 Results and discussion
The influence of different pH media on treated peat has been investigated and the results, for more clarification, in terms of the ratio of shear strength of samples cured in different pH media to shear strength of samples in distilled water versus time, for fibrous, hemic and sapric peats are presented in Fig.2. A ratio of one indicates no effect on cementing process; greater than one indicates an improvement in the cement setting; less than one indicates a negative effect in the cementing process. The shear strength of three samples was determined for each category, and the average shear strength value was used in calculating the strength ratio. The values of shear strength in media with different pH values versus the shear strength in distilled water is shown in Table 2. The moisture content of the samples and the time of curing are also shown in Table 2.
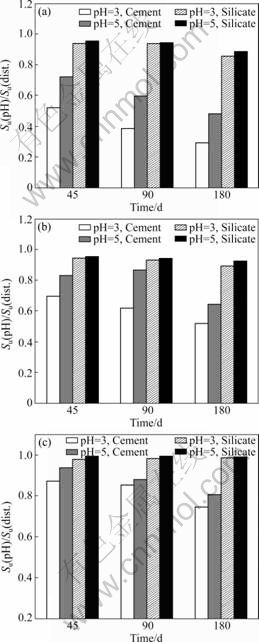
Fig.2 Undrained shear strength ratios of different peats: (a) Fibrous; (b) Hemic; (c) Sapric
It is observed that with low pH (3 and 5), the shear strength of all the samples (fibrous, hemic and sapric) shows a substantial decrease with curing time. The strength ratio of cement treated fibrous peat in media with pH=3 is 0.52 after 45 d, and it is reduced to 0.3 after 180 d of curing. The decreases in strength ratio are 42%, 28% and 14% for fibrous, hemic, and sapric peats, respectively, at pH=3, and treated with cement. The decrease occurs up to 90 d of curing for fibrous peat, but becomes gradual for hemic and sapric peats. The samples cured in media with pH=5 show a higher shear strength compared with the samples cured in media with pH=3 with stabilizer and curing time remaining the same. A similar decrease in the strength ratio is observed with other media and treated with cement or sodium silicate. This clearly leads to the fact that acidic peat nature is harmful to the cementation and pozzolanic activity.
Table 2 Values of shear strength in media with different pH values, moisture content of samples and time of curing

When peat particles react with cement, cation exchange and flocculation take place and these are responsible for the improvements in strength and load-deformation behavior. The cement produces free calcium cations (Ca++) when it comes into contact with water and replaces dissimilar adsorbed cations on the colloidal surface [23-24]. Practically, all fine-grained soils display rapid cation exchange and flocculation- agglomeration reactions when treated with cement in the presence of water. The shear strength increase for the sapric peat is higher in comparison with that of the hemic and fibrous peats. As shown in Table 1, CEC for the sapric peat (82 meq/100 g) is higher than that for the hemic (68 meq/ 100 g) and fibrous (61 meq/100 g) peats, and the most likely mechanism is the cation exchange or crowding of additional cations onto the peat particles. The factors affecting stabilized organic soil such as peat depend upon: water content; physical, chemical and mineralogical properties; nature and amount of organic content, and pH of pore water. TREMBLAY et al [25] reported that the properties of organic soils treated by cement depend not only on the content of the organic matter but also on the nature or the type of the organic matter. The strength gained will also depend upon the decomposition of the organic compound to organic acid due to the biological activity. The engineering behavior of fine-grained soils is mostly influenced by their specific surface area [26]. The specific surface areas of the sapric, hemic and fibrous peats are 93 m2/g, 69 m2/g and 50m2/g, respectively. As the specific surface of peat increases, a greater surface area is available (sapric peat) for reaction in a unit mass or volume basis, hence higher shear strength compared with other two peats appears.
The effect of aggressive media on the silicate confection is not tangible due to the chemical reaction taking place in it. Even as the confection is far from being supersaturated with Ca++ ions, the clinker minerals contained in the confection are intensively hydrated and the OH- ions pass into the solution to be consumed there for depolymerization and hydrolysis of the silicate anions of the additive and for an increase in the pH value of the liquid phase. During this period, the hydrated calcium silicates are formed both via the precipitation of silicate ions of the additive and via the release of silicate and aluminate ions from the clinker [27].
In addition, as mentioned earlier, sodium silicate is basic and will be precipitated as a gel by neutralization. Thus, a dilute sodium silicate solution mixed with certain acids or acid salts will form a gel after a time interval, related to the chemical concentrations [26].
The negative effects of low pH (3 and 5) on the fibrous peat are tangible and more on the hemic and sapric treated peat. This behavior of the fibrous peat is for the reason that, high organic content, high moisture content, high permeability, and low pH (Table 1) in the fibrous peat inhibit the hydration compared with the other peats. Acidic media (pH=3 and 5) during curing result in easy contact with cement in the fibrous peat and inhibit the hydration and pozzolanic reactions.
CHEN and WANG [28] explained that the pH value of the pore solution is important for hydration. When the value is lower than 9, the hydration products are dissolved, producing no hardening or low hardening. If acid in peat is not neutralized by sufficient binder, the acid strongly retards the hydration and secondary pozzolanic reactions because it has a strong chemical affinity to calcium liberated from the cement hydrolysis.
The variation in moisture content with time during the curing process is shown in Fig.2. The moisture content of cement treated fibrous peat is 178% after 45 d of curing, and shows a very small reduction even after 180 d of curing. It is observed that the moisture content of all the samples (fibrous, hemic, and sapric) with cement or silicate is influenced during the curing period and the moisture content of all the samples shows a very small reduction (maximum 6%) up to 90 d of curing. Beyond this period, it remains constant for the fibrous peat (Fig.3(a)), but for the hemic (Fig.3(b)) and sapric (Fig.3(c)) peats, it has a small gradual reduction (no more than 1.5%).
After the initial hydration reaction, it is seen that the physico-chemical reaction of cement and colloidal particles present in the peat has an influence on the flocculation. This is the reason why there is not much difference in the bulk density despite using cement. But, the cement hydration reduces the water content of the stabilized peat and produces solid products of cementation and pozzolanic reactions. Hence, there is an increase in the density of the stabilized soil and a decrease in the moisture content of mixture with curing.
This agrees well with the findings of GAO and ZHOU [29].
The microstructural effects of cementation on the fibrous and sapric peats in distilled water are shown in Figs.4(a) and (b). It can be seen that massive and spherical or coralloid aggregation is formed in the samples.
Furthermore, there are few spherical C-S-H particles or other types of C-S-H morphologies of cement which exhibit less nominal hydration in the fibrous peat than the sapric peat. This is for the reason that higher C-S-H gel is produced in distilled water (denser, almost in spherical form) and a denser paste is formed in comparison with pH=3 (Figs.5(a) and (b)) [30-31].
Furthermore, the microstructure of peats does not change much with silicates, and sodium hydro silicate gel is produced in the same way as that in the aggressive media for treated sapric and fibrous peats (Figs.6(a) and (b)). However, the C-S-H gel in the treated sapric peat is observed to be higher than that in the treated fibrous peat as discussed earlier. The SEM results agree well with the UCS results that the shear strength of samples is less in low pH media, due to less production of C-S-H gel.
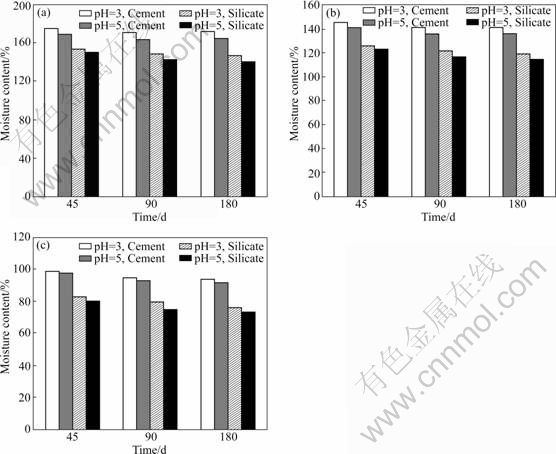
Fig.3 Moisture content versus time of different peats: (a) Fibrous; (b) Hemic; (c) Sapric

Fig.4 SEM images of cement treated peat cured in different media: (a) Fibrous peat in distilled water; (b) Sapric peat in distilled water

Fig.5 SEM images of cement treated peat cured in different media: (a) Fibrous peat in pH=3; (b) Sapric peat in pH=3
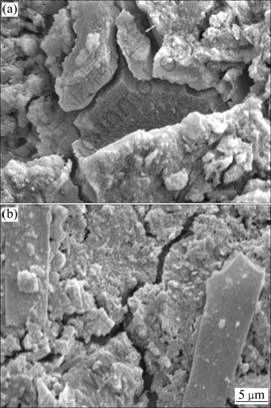
Fig.6 SEM images of silicate treated peat cured in different media: (a) Fibrous peat in pH=3; (b) Sapric peat in pH=3
4 Conclusions
1) Acidic media have the actual percentage loss on the strength of cement treated peats. Aggressive media do not have a significant actual percentage loss on the silicate treated peat due to its high alkaline media.
2) The effect of cement and silicate are insignificant on the fibrous peat among other peats, because of high organic content and low degree of humification, low CEC, low surface area, high permeability, and low colloidal particles in fibrous peat.
3) Silicate and cement have a larger effect on the sapric peat than fibrous peat, which establishes strong chemical reactions in the silicate treated peat, and also high cementation and pozzolanic reactions.
4) The microstructural changes in the treated peats establish the UCS results and the effects of aggressive pH media.
Acknowledgment
The authors wish to express their gratitude to the Ministry of Science, Technology Innovation, Malaysia (Project No. 03-01-04-SF0889) for the financial support of this research.
References
[1] KAZEMIAN S, HUAT B B K, PRASAD A, BARGHCHI M. A state of art review of peat from general perspective [J]. International Journal of the Physical Sciences, 2011. (in Press).
[2] von POST L. Geological survey of Sweden peat inventory and some of its hitherto missing data [J]. Tidskr, 1922, 1: 1-27.
[3] American Society for Testing. Materials Annual: Annual book of ASTM standards [M]. Philadelphia, PA, USA, 1992: 04.08.
[4] EDIL T B, WANG X. Shear strength and K0 of peats and organic soils [M]. Geotechnics of High Water Content Materials, ASTM STP 1374, West Conshohocken, Pa., 2000: 209-225.
[5] CHARMAN D. Peatlands and environmental change [M]. Chichester, UK, John Wiley & Sons Ltd, 2000: 190-200.
[6] ROSS S M. Organic matter in tropical soils: Current conditions, concerns and prospects for conservation [J]. Progress in Physical Geography, 1993, 17(3): 265-305.
[7] ANDRIESSE J P. Nature and management of tropical peat soils [M]// FAO Soils Bulletin 59. Rome, Italy, 1988: 1-165.
[8] ANDERSON C D, SUDOL E D, EL-AASSE M S. Elucidation of the miniemulsion stabilization mechanism and polymerization kinetics [J]. Journal of Applied Polymer Science, 2003, 90(14): 3987-3993.
[9] EUROSOILSTAB. Development of design and construction methods to stabilize soft organic soils: Design guide soft soil stabilization [S]. CT97-0351. Industrial & Materials Technologies Programme (Brite- EuRam III), European Commission, 2002: 15-60.
[10] HEBIB S, FARRELL E R. Some experiences on the stabilization of Irish peats [J]. Can Geotech J, 2003, 40(1): 107-120.
[11] HOLM G. Deep stabilization by admixtures [C]// Proc 13th Int Conf on Soil Mech and Foundation Eng. New Delhi, India, 1994: 161-162.
[12] JELISIC N, LEPPANEN M. Mass stabilization of organic soils and soft clay [M]. Reston, VA, USA: Geotechnical Special Publication (120 I), 2003: 552-561.
[13] AXELSSON K, JOHANSSON S, ANDERSSON R. Stabilization of organic soil by cement- and puzzolanic reactions–feasibility study [R]. Link?ping, Sweden, Swedish Deep Stabilization Research Center, 2000.
[14] KAROL R H. Chemical grouts and their properties [M]. BAKER W H, Ed. Grouting in Geotechnical Engineering, American Society of Civil Engineering, USA, 1982: 359-377.
[15] KAROL R H. Chemical grouting and soil stabilization [M]. New Jersey, USA, Marcel Dekker Inc, 2003: 15-65.
[16] CORPORATION P Q. Soluble silicate in geotechnical grouting applications [M]. 2003, Bulletin 52-53, U.S.A.
[17] SHROFF A V, SHAH D L. Grouting technology in tunneling and dam construction [M]. Balkema, Rotterdam, Netherlands, 1999: 10-59.
[18] US ARMY CORPS OF ENGINEERS. Chemical grouting [R]. USACE Manual No. 1110-1-3500, Washington D C, USA, 1995.
[19] CIRIA. Grouting for grouting engineering [M]. London, UK: CRIRIA Press, 2000: 17-22.
[20] British Standard Institution. Methods of test for soils for civil engineering purposes [S]. London, UK, BS 1377- 1990: Part 2, 3, 6 and 7, 1990.
[21] GILLMAN G P, SUMPTER E A. Modification to compulsive exchange method for measuring exchange characteristics of soils [J]. Aust J Soil Res, 1986, 24: 61-66.
[22] BRUNAUER S, EMMETT P H, TELLER E. Adsorption of gases in multimolecular layers [J]. J of American Chemical Society, 1938, 60: 309-319.
[23] STEVENSON F J. Humus chemistry, genesis, composition, reactions [M]. US, John Willy & Sons Inc., 1994: 429-459.
[24] WANG Hai-dong, QIU Guan-zhou, HUANG Sheng-sheng. Cement industry control system based on multi agent [J]. J Cent South Univ Technol, 2004, 11(1): 41-45.
[25] TREMBLAY H, DUCHESNE J, LOCAT J, LEROUEIL S. Influence of the nature of organic compounds on fine soil stabilization with cement [J]. Can Geotech J, 2002, 39: 535-546.
[26] SANTAMARINA J C, KLEIN K A, WANG Y H, PRENCKE E. Specific surface area: Determination and relevance [J]. Can Geotech J, 2002, 39: 233-241.
[27] BRYKOV A S, DANILOV B V, LARICHKOV A V. Specific features of Portland cement hydration in the presence of sodium hydrosilicates [J]. Russian Journal of Applied Chemistry, 2006, 79(4): 521-524.
[28] CHEN H, WANG Q. The behavior of organic matter in the process of soft soil stabilization using cement [J]. Bull Eng Geol Environ, 2006, 65: 445-448.
[29] GAOYing-li, ZHOUShi-qiong. Influence of ultra-fine fly ash on hydration shrinkage of cement paste [J]. J Cent South Univ Technol, 2007, 12(5): 596-600.
[30] KAZEMIAN S, HUAT B B K, BOLOURI BAZAZ J, ABDUL AZIZ F N, MOHAMMED T A. Influence of the peat characteristics on cementation and pozzolanic reactions in dry mixing method [J]. The Arabian Journal for Science and Engineering, 2011. (in Press)
[31] KAZEMIAN S, HUAT B B K, PRASAD A, BARGHCHI M. Study of peat media on stabilization of peat by traditional binders [J]. International Journal of Physical Sciences, 2011, 6(3): 476-481.
(Edited by YANG Bing)
Received date: 2010-08-02; Accepted date: 2010-11-01
Corresponding author: S. KAZEMIAN, PhD; Tel: +60-17-39394040; E-mail: sina.kazemian@gmail.com

