
Adsorption behavior and mechanism of amino methylene phosphonic
acid resin for Ag(Ⅰ)
Shu Zeng-Nian(舒增年)1, Xiong Chun-Hua(熊春华)2, WANG Xu(王 旭)1
1. Department of Chemistry, Lishui University, Lishui 323000, China;
2. College of Food Science, Biotechnology and Environmental Engineering, Zhejiang Gongshang University,
Hangzhou 310035, China
Received 1 August 2005; accepted 12 January 2006
Abstract: The sorption properties of amino methylene phosphonic resin(APAR) for Ag(Ⅰ) were studied. The amino methylene phosphonic acid resin(APAR) has a good adsorption ability for Ag(Ⅰ) at pH=6.0 in the HAc-NaAc medium. The statically saturated adsorption capacity is 272 mg/g resin, Ag(Ⅰ) adsorbed on APAR can be eluted by 5% (NH2)2CS-0.5 mol/L hydrochloric acid quantitatively. The adsorption rate constants determined under various temperatures are k15℃=3.89×10-5 s-1, k25℃=5.93×10-5 s-1, k35℃=7.59×10-5 s-1, k45℃=9.45×10-5 s-1, respectively. The apparent activation energy of adsorption, Ea is 22.8 kJ/mol, the enthalpy change (?H) of sorption is 17.4 kJ/mol. The adsorption mechanism shows that the functional group of APAR coordinates with Ag(Ⅰ) to form coordination bond, the coordination molar ratio of the functional group of APAR to Ag(Ⅰ) is 1∶1.
Key words: amino methylene phosphonic acid resin(APAR); Ag; adsorption; mechanism
1 Introduction
Many efforts have been made on using ion exchange resin to separate and concentrate certain metal cations [1-11]. However, less research for using it to adsorb noble metal Ag(Ⅰ), and much less research for commercializa- tion has been done so far. Since Ag has many applications, it will be important to synthesize and select the ion exchange resin, which has high adsorption capacity and easy desorption capacity for noble metal Ag(Ⅰ). Amino methylene phosphonic acid resin (APAR) is a novel chelate resin, and contains a functional group of [—CH2NHCH2PO(OH)2], which possess not only proton that can exchange with cation, but also oxygen and nitrogen atoms that can coordinate directly with metal ion and form stable coordination compound. In recent years, chelate resin containing phosphorous atoms, which adsorb certain metal ions, has become more and more active research topic. But no research and experiments of APAR absorbing Ag(Ⅰ) have been reported so far. In this article, we described the adsorption behavior and mechanism of APAR with Ag(Ⅰ). The basic adsorption parameters were determined for the first time. The experimental results may be applied to concentration and extraction of Ag(Ⅰ) in hydro metallurgy.
2 Experimental
2.1 Materials and instruments
Instruments: HZ9212s temperature constant shaking machine (±0.1 ℃), 722 spectrophotometer, Sartorius PB- 20 pH meter, Elemental Analyzer EA1110 and Perkin- Elmer 683 FT-IR.
Materials: amino methylene phosphonic acid resin (provided by Nankai University); standard solution of Ag(Ⅰ) was prepared from AgNO3; 0.03% rhodanine acetone solution; 1% Arabic gum solution(compounding fresh), buffer solution with pH 2.63-6.2 from HAc-NaAc, other reagents were A.R grade.
2.2 Sorption and analytical method of resin
Static sorption equilibrium experiment: a desired amount of treated resin was weighed and added into a conical flask, then a desired volume of the buffer solution was added. After 24 h, a required amount of standard solution of Ag(Ⅰ) was added. The flask was shaken in a shaker at constant temperature. The upper layer of clear solution was taken for analysis until a sorption equilibrium was reached, the amount of Ag(Ⅰ) was measured by the specteophotometric determination of rhodanine. The distribution coefficient (D) and the adsorption amount (QR) were calculated with the formulas:
D=QR/ρe (1)
QR=(ρ0-ρe)V/m (2)
where QR is the adsorption amount of APAR to metal ion in equilibrium state, mg?g-1; ρo is the initial concentration of metal ion in solution, mg?mL-1; ρe is the equilibrium concentration of metal ion in solution, mg?mL-1; m is the resin mass, g; V is the total volume of solution, mL.
3 Results and discussion
3.1 Effect of pH on distribution coefficient
The test was carried out according to the method mentioned above. Seven parts of 20.0 mg resins were weighed and put into conical flasks individually. The effect of pH value on the adsorption behavior of APAR for Ag+ in HAc-NaAc buffer solution is shown in Fig.1. The results indicate that the distribution coefficient increases with the increase of pH value. The distribution coefficient is the highest at pH 6.0, and then almost keeps constant when pH is over 6.0. In order to prevent Ag+ from hydrolyzing, all the following experiments were performed at pH =6.0.
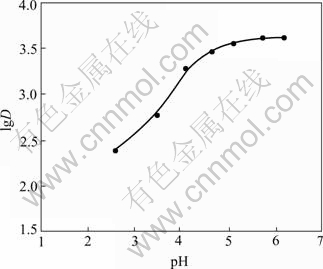
Fig.1 Effect of pH on distribution coefficient (Resin 20.0 mg; [Ag+]0=0.22 mg/mL; T=298 K; total volume of solution 30.0 mL)
3.2 Isotherm adsorption curve
10.0, 15.0, 20.0, 25.0 and 30.0 mg of resin were weighted and put into conical flasks individually. The experimental conditions are shown in Fig.2. When the adsorption equilibrium is reached, equilibrium concen- tration(ρe ) is determined and the corresponding adsorp- tion capacity of APAR Q(mg/g) is calculated and plotted in Fig.2. The adsorption isotherm is further correlated to the well-known Freundlich equation:
lgQ=(1/n)lgρ+lgk (3)

Fig.2 Freundlich isotherm curve of sorption Ag(Ⅰ) ([Ag+]0=0.20 mg/mL; T=298 K; total volume of solution 30.0 mL)
According to lgQ—lgρ, the coefficient straight line is obtained, the correlation coefficient of straight line (r =0.996 3) is achieved. Freundlich “n” value is 2.50. The fact that n value is between 2 and 10 which indicates that Ag(Ⅰ) is easy to be absorbed[12].
3.3 Determination of adsorption rate constant
40.0 mg resin was weighed accurately, according to the experimental conditions of T=298 K, [Ag+]=0.25 mg/mL and the method mentioned previously, 0.25 mL of the upper layer clear solution was taken out at intervals for the determination of remaining concentrations, after the remains kept constant and volume was corrected, a series of data were obtained. When the adsorption amount is half of that at equilibrium, the required time t? is about 2 h. The required time of the sorption equilibrium is 14 h. According to Ref.[13], the sorption rate constant k can be calculated from -ln(1-F)=kt, where F=Qt /Q∞, and Qt and Q∞ are the sorption amounts at sorption time and at equilibrium, respectively. The slope of straight line which is plotting -ln(1-F) versus, yields the sorption rate constant k which is k298=5.93×10-5s-1. The correlation coefficient (r=0.995 9) was obtained via linear fitting. According to BOYD[14] from the linear relationship of -ln(1-F)—t, it can be deduced that the liquid film spreading is the predominating step of the sorption process.
According to the experimental condition shown in Fig.3, the experiments were carried out by using the above-mentioned method at 15, 25, 35 and 45 ℃, respectively, and a series of data about the sorption amounts and the certain time are obtained. The experimental results are shown by plotting -ln(1-F) vs t (Fig.4). Therefore, the sorption rate constant or the sorption rate constant of APAR for Ag(Ⅰ) can be found from the slope of the straight line. That is shown in Table 1.
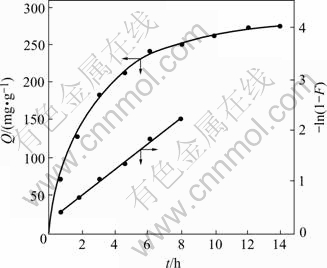
Fig.3 Sorption rate curve of Ag(Ⅰ) (Resin 40.0 mg; [Ag+]0= 0.25 mg/mL; T=298 K; total volume of solution 60.0 mL)
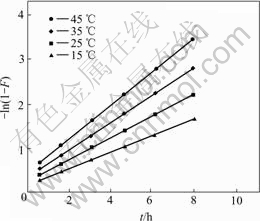
Fig.4 Determination of adsorption rate constant between 288 and 315 K (Resin 40.0 mg; [Ag+]0= 0.25 mg/mL; total volume of solution 60.0 mL)
Table 1 Sorption rate constants of APAR for Ag(Ⅰ) under various temperatures
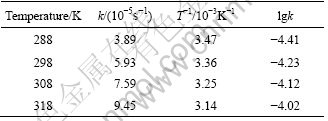
According to the formula of Arrhenius lnk=-Ea/RT+lnA and the data in Table1, the slope of straight line, which is made by plotting lgk versus 1/T (Fig.5), and calculated by linear fitting, yields the sorption rate constant of -1.19×103, and the correlation coefficient R of 0.993 7, and the apparent activation energy of Ea=22.8 kJ/mol. It can be seen from the rate constant that, the sorption speed can be raised about double, when the temperature is raised by 20 ℃ within the scope of experimental temperature.
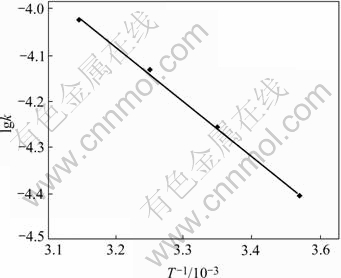
Fig.5 Determination of adsorption apparent activation energy (Experiment condition is the same as Fig.4)
3.4 Influence of sorption temperature on sorption percentage and determination of thermodynamic parameters
Four parts of 20.0 mg resins were weighed. Under the experimental conditions as shown in Fig.6, the distribution ratio D of the resin for Ag(Ⅰ) is determined, over the temperature range of 289-313 K. From the result shown in Fig.6, it can be seen that the increasing sorption temperature leads to better sorption. This means that the sorption process is an endothermic process. Therefore, the sorption reaction is a chemical sorption. The intercept is 8.23, the slope of the straight line in Fig.6 is K=-0.91×103, According to lgD=-?H/ 2.303RT+?S/R, ?H value of 17.4 kJ/mol is obtained, ?S can be obtained from the intercept of the line, which is 68.4 J/(mol?K), the sorption reaction of Ag(Ⅰ) is a spontaneous reaction under the experimental condition of T=298 K, and the result, ?G298=?H-T?S=-2.97 kJ/mol.
3.5 Determination of complex ratio
1) Saturated capacity method
40.0 mg of resin was weighed accurately. Under the experimental conditions of T=298 K, [Ag+]=0.20 mg/mL, the experiment was performed by using the above-mentioned method. The sorption capacity of resin for Ag+ is 272 mg/g, i.e. 2.52 mmol/g. The amount of functional group is 2.59 mmol/g. The molar ratio of the functional group to Ag(Ⅰ) is 1.03∶1, which is 1∶1 approximately.
2) Equimolar method
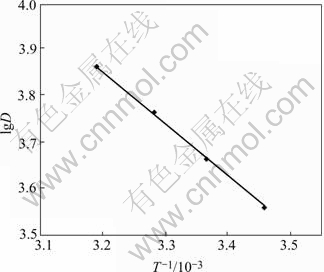
Fig.6 Effect of temperature on distribution coefficient (Resin 20.0 mg; [Ag+]0=0.20 mg/mL; total volume of solution 30.0 mL)
Seven parts of different amounts of resins were accurately weighted and added into the different conical flasks, then mixed with different amounts of Ag(Ⅰ). The total APAR functional group and Ag(Ⅰ) were kept at 135.0 mmol whatever the molar ratio might be. The experiment was carried out with the same method mentioned previously.
The curve of sorption amounts vs [Ag+]/([Ag+]+[R]) is shown (units of formula is mmol, R is APAR’s functional group) in Fig.7. The expected sorption amount is the biggest where the molar fraction of Ag(Ⅰ) is 0.51. This means that the complex molar ratio of the functional group to Ag(Ⅰ) is about 0.96∶1, which is 1∶1 approximately. The result is consistent with the result obtained from saturated capacity method.
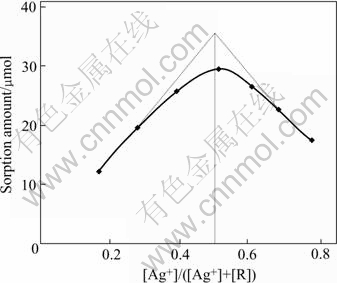
Fig.7 Equimolar series method (Total volume of each solution 30.0 mL, T=298 K)
3.6 Analysis of infrared spectra of sorption of resin for Ag(Ⅰ)
In order to make further approaching of the functional group of resin and Ag(Ⅰ), the spectra of resin, before and after Ag(Ⅰ) is adsorbed, are compared. It is found that the characteristic sorption peaks of the bonds N—H shift from 3 410 cm-1 to 3 375 cm-1 and when the bonds P=0 is at 1 175 cm-1, the sorption peaks disappear on the whole, which shows that the formation of the coordination bonds between nitrogen and oxygen atoms and Ag(Ⅰ) weakens the stretch vibration and causes the peak to shift to the lower frequency. The characteristic sorption peak of P—OH (930 cm-1) is weakened and the new characteristic sorption peak of (—PO3-2) is formed[15], which shows that H and Ag+ has been exchanged. All those changes result from the formation of complex compound.
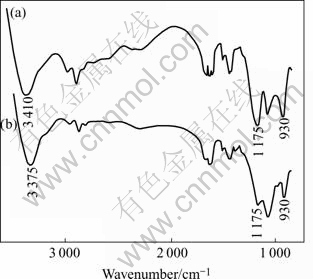
Fig.8 IR spectra of APAR resin: (a) Before adsorption; (b) After adsorption
3.7 Elution and recovery of resin
Four parts of 30.0 mg resin were accurately weighted and added into different conical flasks at T=25℃, [Ag+]0=0.20 mg/mL. The flasks were shaken until the upper layers of clear solution were taken for analysis and sorption equilibriums were reached. The sorption amount was calculated individually. The separated resin from residual aqueous phase was washed three times with the buffer solution of pH=6.0 HAc-NaAc. After the resin were dried, daunts ammonia, thiourea, sodium thiosulphate and thiourea-hydrochloric acid were added into the resin respectively. The result of elution shows that the system of 5% thiourea-0.5 mol/L HCl is most efficient. The percentage of elution is up to 93% for the first time. Two times it is up to 99%. The sorption capacity of the resin is almost not changeable and the APAR can be regenerated. The resin can be regenerated and reused. Therefore 5% thiourea-0.5 mol/L HCl can be used as eluant to collect Ag. In fact, [Ag+] adsorbed by APAR and thiourea hydrochloric acid result from the formation of complex compound and was eluted[16].
4 Conclusions
1) Results of the adsorption experiment show that Ag(Ⅰ) can be optimally adsorbed on amino methylene phosphoric acid resin in the HAc-NaAc system at pH=6.0, the statically saturated adsorption capacity is 272 mg/g resin, The Ag+ adsorbed on APAR can be eluted by using 5% thiourea-0.5 mol/L HCl quantita- tively. The two times percentage of elutions adds up to 99%.
2) The adsorption behavior of APAR for Ag(Ⅰ) obeys the Freundlich isotherm and n value is between 2 and 10, which indicates that Ag(Ⅰ) is easy to be adsorbed. The apparent adsorption rate constant is k298=5.95×10-5 s-1, and the thermodynamic adsorption parameters are ?H=17.4 kJ/mol, ?G=-2.97 kJ/mol, Ea=22.8 kJ/mol.
3) The molar ratio of the functional group to Ag(Ⅰ) is approximately 1∶1 by saturated capacity method or equimolar method.
4) After APAR’s adsorption of Ag(Ⅰ), some characteristic absorption peaks of group are changed. This show that H of APAR’s functional group exchanged with Ag(Ⅰ) and formed coordination compound.
References
[1] XIONG Chun-hua, SHU Zeng-nian, CHEN Yi-yong. Studies on the sorption of macroporous phosphonic acid resin for lanthanum [J]. Chinese Journal of Reactive Polymer, 1998, 7(2): 7-15.
[2] SHU Zeng-nian, XIONG Chun-hua, LIN Feng. Studies On the adsorption of yhrium(Ⅲ) by macroporous phasphonic acid resin [J]. Chemical Research and Application, 1997, 9(3): 289-293. (in Chinese)
[3] CHEN Yi-yong, LIANG Chao, CHAO Yan. Synthesis and characterization of polyacrylonitrile2thiosemicarbazide resin and its sorption behavior for rh(Ⅲ) ru(Ⅳ) pd(Ⅱ) and ir(Ⅳ) ions [J]. Reactive Polymers, 1998(36): 51-58.
[4] XIONG Chun-hua, SHU Zeng-nian, WANG Yong-jiang. Sorption of Mo(Ⅵ) by 4-aminopyridine resin [J]. Journal of Chemical Industry and Engineering, 2005, 56(7): 1267-1270. (in Chinese)
[5] FAN Cai-mei, MIN Yan-qin, HAO Xiao-gang, SUN Yan-ping. Adsorption and photocatalytic degradation of phenol over TiO2/ACF [J]. Trans Nonferrous Met Soc China, 2003, 13(2): 452-456.
[6] XIONG Chun-hua, WU Xiang-mei. Adsorption of copper using macroporous phosphonic acid resin [J]. Trans Nonferrous Met Soc China, 2003, 13(6): 1446-1450.
[7] XIONG Chun-hua, WU Xiang-mei. Study on the adsorption of iminodiacetic acid resin for yttrium(Ⅲ) [J]. Chinese Journal of Inorganic Chemistry, 2003, 19(12): 1356-1360.(in Chinese)
[8] XIONG Chun-hua, WU Xiang-mei. Studies on the sorption behavior and mechanism of macroporous phosphonic acid resin for cadmium(Ⅱ) [J]. Acta Scientiae Circumstantiae, 2000, 20(5): 627-630.
[9] XIONG Chun-hua, SHEN Qiu-xian. Adsorption of Er(Ⅲ) and its mechanism on diglycolamidic acid resin [J]. Journal of Rare Earths, 2002, 20(5): 492-496.
[10] LI Wei-shi, SHEN Zhi-quan, ZHANG Yi-feng. Studied on new type chelating resins(I) [J]. Chemical Journal of Chinese Universities, 1998, 19(2): 322-325. (in Chinese)
[11] XIONG Chun-hua, SHU Zeng-nian. Adsorption behavior and mechanism of 4-amino-1,2,4-triazole resin for molybdenum(Ⅵ) [J]. Nonferrous Metals, 2000, 52(2): 61-64. (in Chinese)
[12] HIRO K, RENICHIROU S. Foundation and Design of Adsorptions [M]. LU Zheng-li trans. Beijing: Chemical Industry Press, 1983. 33-36. (in Chinese)
[13] BRYKINA G D, MARCHAK T V, KRYSINA L S, BELYAVSKAYA T A. Sorption-photometric determination of copper by using AV-17 anion exchanger modified with 1-(2-thiazolyl-azo )-2-naphthol-3, 6-disulphonic acid [J] . Zh Anal Khim, 1980, 35(12): 2294-2299.
[14] BOYD G. E,ADAMSON A W, MYERS L S. The exchange adsorption of ions from aqueous solutions by organic zeolites Ⅱ kinetics [J]. J Am Chem Soc, 1947, 69: 2836-2848.
[15] MA Ai-zeng, LI Lai-ming, ZENG Guang-bin, WANG Cui-ying, LI Han. The IR spectral studies on rare earths HEH[EHP] with glycine conplexes [J]. Chinese Journal of Applied Chemistry, 1989, 6(1): 41-45. (in Chinese)
[16] HE Qi. The synthesis of thiourea polymeric resin beads and the research on its capability for adsorption Ag(Ⅰ) [J]. Ion Exchange and Adsorption, 1991, 7(1): 42-46.
Foundation item: Project(20040501) supported by the Department of Education of Zhejiang Province, China
Corresponding author: SHU Zeng-nian; Tel: +86-578-2271338; E-mail: zengnianshu@yahoo.com.cn
(Edited by LI Xiang-qun)