Article ID: 1003-6326(2005)03-0491-06
Oxidation behavior of three-phase Cu-25Ni-30Cr alloy at 700-800℃ under high oxygen pressures
CAO Zhong-qiu(曹中秋)1, 2, LIU Wei-hua(刘伟华)1,
ZHAI Guang-ping(翟广平)1, NIU Yan(牛 焱)2
(1. Department of Chemistry, Shenyang Normal University,
Shenyang 110034, China;
2. State Key Laboratory for Corrosion and Protection, Institute of Metal Research,
Chinese Academy of Sciences, Shenyang 110016, China)
Abstract: The thermogravimetric analysis of a ternary Cu-25Ni-30Cr alloy prepared by conventional casting was performed in 0.1MPa pure O2 at 700-800℃. The results show that the alloy is composed of three phases, where the phase with the largest copper and lowest chromium content forms the matrix, while the other two, much richer in chromium, form a dispersion of isolated particles. At variance with another three-phase Cu-20Ni-20Cr alloy, which forms complex scales containing the oxides of the various components and double oxides plus an irregular region composed of alloy and oxides, the present alloy can form a very irregular but continuous chromia layer at the base of the mixed internal region, producing a gradual decrease of the oxidation rate down to very low values. A larger chromium content needed to form chromia layer for a ternary three-phase alloy is attributed to the limitations to the diffusion of the alloy components in the metal substrate imposed by their multiphase nature.
Key words: Cu-Ni-Cr alloy; three-phase; high-temperature oxidation CLC
number: TG174 Document code: A
1 INTRODUCTION
Materials and/or coatings used for technological applications at high temperatures often contain many components and more than one phase, usually require to provide mechanical properties and oxidation resistance sufficient to ensure a reasonably long life under working conditions[1, 2]. In fact, it is necessary to form external and slowly-growing Cr2O3, Al2O3 and SiO2 scales on the alloy surface by adding Cr, Al and Si elements to alloys in order to protect these alloys against oxidation at relatively high temperatures. A lot of studies have proved that the formation of external scales of the most stable oxide for binary two-phase alloys is more difficult than that for solid-solution alloys under the same values of all the parameters involved, as predicted[3-12] and also observed experimentally[13-17]. For ternary multi-phase alloys, no systematic analysis of the scaling behavior has been developed so far, in spite of their technological importance, mainly as a consequence of the larger variety of possible scaling modes and of the many importance complication concerning various aspects, such as the thermodynamic properties of the alloy components and of their compounds with the oxidant as well as the diffusion processes in the alloy and in the scale with respect to the simpler case of binary alloys[2, 18]. Even though more complex, the oxidation behavior of ternary multi-phase alloys is also expected to be similar to that of binary two-phase alloys.
Copper, nickel and chromium form oxides whose thermodynamic stability increases in the given order. Moreover, copper and nickel are completely soluble, while nickel can dissolve chromium up to a large content. Finally, copper and chromium are very slightly soluble into each other and do not form any intermediate phase[19]. Therefore Cu-Ni-Cr alloys can be used as model materials to study the fundamental oxidation mechanisms of ternary multi-phase systems. NIU et al[14] widely investigated the oxidation behavior of binary two-phase Cu-Cr alloys and concluded that a 75%(mass fraction) chromium content is not yet sufficient to form a chromia layer on the alloy surface. CAO et al[20] investigated the oxidation behavior of the three-phase Cu-20Ni-20Cr alloy by adding Ni to Cu-Cr alloy and concluded that the alloy can not form continuous chromia layer even after extended oxidation periods. On the contrary, it forms complex scales containing mixtures of the oxides of the various components and double oxides plus an irregular region composed of a mixture of alloy and oxides, which does not correspond to the classical case of internal oxidation, while the alloy/scale interface is very irregular. The aim of this work is to examine the oxidation behavior of a three-phase Cu-Ni-Cr alloy containing 30%(mole fraction) chromium (denoted as Cu-25Ni-30Cr) to establish the possibility of formation of continuous chromia layer in spite of the simultaneous presence of three different phases.
2 EXPERIMENTAL
A Cu-25Ni-30Cr alloy was prepared by arc-melting appropriate mixtures of the pure components (99.99%) under a Ti-gettered argon atmosphere using non-consumable tungsten electrodes. The alloy ingot was subsequently annealed in vacuum at 800℃ for 24h to remove residual mechanical stresses. The actual average composition of the alloy is 25.8%(mole fraction)Ni and 30.5%Cr, balance Cu. The phase diagram of the Cu-Ni-Cr system, known only at 930℃ and above, shows the existence of different phases[19]. The present alloy is actually composed of a mixture of three different phases as shown in Fig.1 whose volume fraction can change locally to some extent. The lightest α phase contains about 81%(mole fraction)Cu and 15%Ni, with only about 4%Cr. The medium gray β phase with a larger chromium content contains approximately 16%Cu, 52%Ni and 32%Cr. Finally, the dark γ phase richest in chromium contains approximately 8%Cu, 27%Ni and 65%Cr. The α phase forms the matrix of the alloy, while the γ phase is present in the form of isolated particles, sometimes aggregated into dendritic formations. The β phase occurs also in the form of isolated
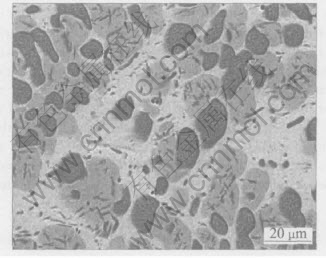
Fig.1 Microstructure of Cu-25Ni-30Cr alloy(BEI)
particles, which sometimes is dispersed in the α matrix, while in other cases surrounds the γ phase particles.
Flat specimens about 1mm thick and with a surface area of about 2cm2 were cut from the alloy ingot by means of diamond-wheel saw, ground down to 600 emery paper, washed in water and acetone and dried immediately before use. The oxidation tests were carried out in 0.1MPa pure O2 at 700-800℃ for 24h with a continuous mass gain measurement using a ThermoCahn Versa HM microbalance. The oxidized specimens were examined by means of X-ray diffraction (XRD), scanning electron microscopy (SEM) and energy-dispersive X-ray microanalysis (EDX) to establish the nature, composition and spatial distribution of the oxidation products.
3 RESULTS
3.1 Oxidation kinetics
The kinetic curves for the oxidation of the present Cu-25Ni-30Cr alloy and the previous Cu-20Ni-20Cr alloy in 0.1MPa pure O2 at 700-800℃ for 24h are shown in Fig.2. For the present Cu-25Ni-30Cr alloy, the oxidation kinetics at 700℃ can be approximately described as three main quasi-parabolic stages, the first with a rate constant kp=2.5×10-11 (all kp values in g2·cm-4·s-1) for the initial 2h, the second with an average kp value of 5.8×10-12 about 10h, and the third with a rate constant kp=4.8×10-13 up to 24h. While the oxidation kinetics at 800℃ is also composed of the three parabolic stages, the first with a rate constant kp=8.6×10-10 for the initial 3h, the second with an average kp value of 1.4×10-10 about 12h and the third with a rate constant kp=5.7×10-11 up to 24h. At both temperatures,
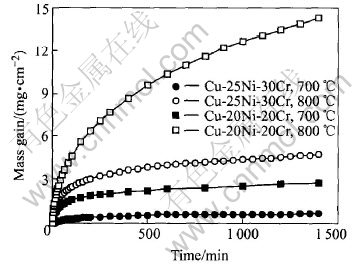
Fig.2 Oxidation kinetics for Cu-25Ni-30Cr and Cu-20Ni-20Cr alloys oxidized in 0.1MPa pure O2 at 700-800℃ for 24h
the oxidation rates decrease continuously with time more rapidly than that required by the parabolic rate law, so that the instantaneous parabolic rate constants, i.e. the instantaneous slope of the plot of the weight squared vs time, are also decreased with time, indicating that the scales become more protective as the reaction time increases. The Cu-25Ni-30Cr alloy at 700℃ oxidizes more slowly than at 800℃. By comparison, the oxidation rates of the present alloy are significantly smaller than that of the previous three-phase Cu-20Ni-20Cr alloy with lower chromium content at both temperatures.
3.2 Scale microstructures and composition
The scale microstructures of the Cu-25Ni-30Cr alloy oxidized in 0.1MPa pure O2 at 700-800℃ for 24h are shown in Fig.3. The X-ray diffraction (XRD) patterns of the oxide scales at 700-800℃ are shown in Fig.4. The scales formed on alloy surface at 700-800℃ are relatively complex. According to the XRD analysis, some peaks for CuO, NiO, Cr2O3 and NiCr2O4 are present. EDX analysis shows that at 700℃, the main fraction of uni- form lighter external scale is composed of almost
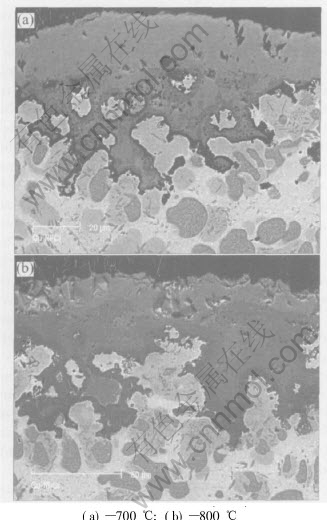
Fig.3 Cross sections of Cu-25Ni-30Cr alloy oxidized in 0.1MPa pure O2 at 700-800℃ for 24h (BEI)
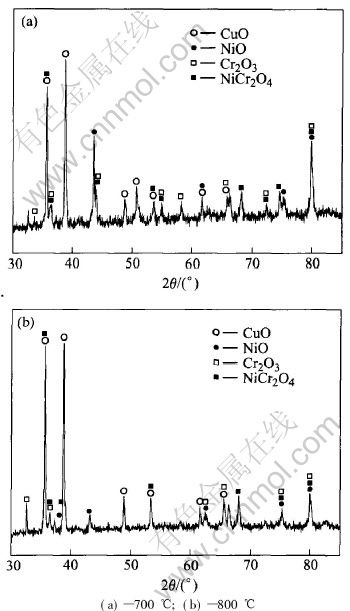
Fig.4 XRD patterns of oxide scales in 0.1MPa pure O2 at 700-800℃ for 24h
pure CuO(73.38%Cu, 2.13%Ni, 0.98%Cr and 23.50%O). This is followed by a region containing deep protrusions into the alloy composed of a thin but discontinuous medium gray NiCr2O4 spinel layer (4.38%Cu, 33.17%Ni, 31.61%Cr and 30.84%O), which also has some β phase particles surrounded by chromia layer, while the innermost irregular dark layer is made of chromia layer (3.16%Cu, 3.13%Ni, 49.94%Cr and 43.76%O). At 800℃, the external scale is relatively uniform and at the same time has some holes, which is composed of almost pure CuO(69.40%Cu, 2.04%Ni, 1.20%Cr and 27.37%O), while the intermediate thick and continuous medium gray layer is made of NiCr2O4 spinel(2.05%Cu, 59.12%Ni, 16.76%Cr and 22.07%O). Finally, the innermost region is made of a continuous dark chromia layer (2.65%Cu, 1.53%Ni, 55.56%Cr and 42.26%O), which is much thicker than that formed at 700℃ on the same alloy. At both temperatures, there is a deep region containing a mixture of alloy and oxide phases. As examined below, this is not a classical case of internal oxidation. In fact, the islands which have been oxidized are not those of the β and γ phases rich in chromium, but those of the α phase presenting quite small chromium content. The β and γ phase islands are surrounded by a dark chromia layer, while the α phase islands have been transformed into NiO containing some copper oxide in solution (slightly darker than CuO) close to the alloy/scale interface, and in a mixture of NiO and copper metal (light) deeper in the alloy. In some cases the chromia layers grown around two close particles are in direct contact, finally, forming a continuous chromia layer, therefore the alloy oxidation rates decrease obviously.
4 DISCUSSION
Previous studies show that the three-phase Cu-20Ni-20Cr alloy can not form a continuous chromia layer over the alloy surface and at the base of the scale even after an extended oxidation periods, so that oxidation involves all the alloy components and proceeds with a rather large rate even if the instantaneous parabolic rate constant decreases with time[20].
At variance with the previous Cu-20Ni-20Cr alloy, the present Cu-25Ni-30Cr alloy also can not produce a regular flat chromia layer directly over the alloy surface in spite of its relatively large chromium content, but can form a continuous chromia layer only within the alloy after an initial transient stage of rather fast oxidation. Thus, a 30%(mole fraction) chromium content is possible to produce a selective oxidation of the most reactive component in spite of the presence of a mixture of three different phases in three-phase Cu-Ni-Cr alloy.
In fact, the approach to predict the oxide scale structure on the alloy surface is based purely on thermodynamic and kinetic considerations. Thermodynamically, the stablities of four various oxides increase in the range from CuO, Cu2O and NiO to Cr2O3, therefore Cr is the most reactive component, while Cu is noble component. Because the oxygen pressure prevailing in the gas is much higher than the equilibrium decomposition pressure, four various oxides all may form when oxidation starts, but Cr2O3 is more stable than CuO, Cu2O and NiO. Kinetically, there are much more differences among the oxidation rates of three components, CuO and Cu2O grow more rapidly than NiO and Cr2O3 on the alloy surface, when oxidation starts, CuO, Cu2O, NiO and Cr2O3 oxides may form, but CuO and Cu2O overlays NiO and Cr2O3 because of rapid growth rate of copper oxides. Thus, a continuous CuO external scales forms initially, which is controlled by the rate of growth of CuO. Alloy/scale interface transfers inward along with oxide scale formation, the oxygen pressure at alloy/scale interface decreases gradually. NiCr2O4 spinel is formed and then oxidation rate is controlled by the rate of growth of NiCr2O4. However, after a chromia layer has formed around the β and γ phase particles, the supply of both copper and nickel to the external scale is blocked and the oxidation rate becomes eventually controlled by the rate of growth of Cr2O3. Therefore oxidation kinetic is composed of three parabolic stages and the instantaneous parabolic rate constant decreases gradually with time to very low value.
The microstructure of the mixed internal region for the present alloy does not correspond to an internal oxidation of the two most reactive components, so this would imply the presence of isolated particles of chromium and nickel oxide within a matrix of pure copper. Moreover, oxygen should penetrate into the alloy by diffusion through the copper matrix and then react with nickel and chromium either at a single or at two different reaction fronts, which should be substantially flat. Contrary to this, oxidation inside the present alloy proceeds mostly along the network of particles of the α phase, while the particles of the β and γ phases, much richer in the most reactive components, remain mostly metallic and are surrounded by a thin but continuous chromia layer which protects their core from further oxidation. Thus, the front between the alloy and the oxidized regions becomes very irregular. Within the α phase islands, both copper and nickel are oxidized in the most external region, while deeper in the alloy copper is present as metal particles in a matrix of nickel oxide. This situation is clearly out of equilibrium, because the β and γ particles presented at the same depth remain unoxidized, indicating that the oxygen activity at their interior is below the stability of chromium oxide, and thus much lower than that prevailing within the oxidized α phase islands. This is a consequence of purely kinetic factors, and more precisely of the presence of a chromia layer around the external surface of the β and γ phase particles, which maintains a low oxygen activity inside these islands and therefore prevents their conversion into oxides.
The thin and irregular chromia layer extends gradually with time to a larger fraction of the inner surface of the mixed region until a continuous chromia layer is eventually established at the base of this region. At this point, the further growth of the less stable oxides of nickel and copper is completely prevented. This process also corresponds to a gradual decrease of the instantaneous parabolic rate constant with time, which eventually will be controlled by the rate of growth of the chromia layer. The final rate of oxidation is extremely small, probably as a consequence of the fact that the chromia layer grows inside the alloy, where the oxygen activity must be very small since the rate of oxygen penetration is quite slow.
A 30%(mole fraction) chromium content is possible to form a continuous chromia layer within a three-phase Cu-25Ni-30Cr alloy after an initial transient stage of rather fast oxidation. For binary two-phase Cu-Cr alloys, the formation of chromia layer is much more difficult, since a 75%(mass fraction) chromium content is not yet sufficient to produce the same oxidation behavior in alloys with a normal grain size[14]. In fact, according to the Gibbs phase rule, a ternary system of the A-B-O type composed of a binary A-B alloy exposed to oxygen can only contain a maximum of three condensed phases in the presence of a gas phase under isothermal conditions, under which conditions the system becomes invariant. Thus, a binary two-phase alloy can be in equilibrium with only one oxide under a single value of the oxygen pressure, while the composition of the two phases must remain constant, there will be no chemical potential gradients to cause long-range diffusion. As a result, diffusion process can hardly occur between difference phases, especially when A and B exhibit a rather limited mutual solubility. A similar situation also applies to oxidation of a ternary multi-phase alloy after making the appropriate modifications. A quaternary system of the A-B-C-O type can only contain a maximum of four condensed phases under isothermal conditions. Thus, a ternary three-phase alloy can be in equilibrium with a single oxide only under a unique value of the oxygen pressure, which is exactly the situation prevailing at the front of internal oxidation. Above this location, the α phase disappears by forming oxides, leaving a surface layer in contact with the scale where the alloy will contain a mixture of only two phases, so that the equilibrium among the three phases is lost and the system becomes mono-variant. In ternary two-phase alloys the two phases can coexist in equilibrium in a finite range of composition, so that their presence does not necessarily prevent the diffusion of the metal components and may decrease critical content needed to form chromia layer. At the same time, the large difference in the critical Cr content needed to form chromia layer in the two systems is very likely a consequence of the larger solubility of chromium in nickel than in copper[20], which allows a more effective diffusion of chromium through the region of internal oxidation to sustain the growth of the chromia layer in the presence of favorable concentration gradients in alloy phases. This behavior is similar to that of binary two-phase alloys, for which the critical content of the most reactive component B needed for its exclusive oxidation decreases significantly as its solubility in the more noble component A increases[5,10,12].
5 CONCLUSIONS
1) The Cu-25Ni-30Cr alloy can not form a regular flat chromia layer directly over the alloy surface in spite of its relatively large chromium content, but can form a continuous chromia layer only within the alloy after an initial transient stage of rather fast oxidation in spite of the presence of a mixture of three different phases in the metal substrate.
2) The main fraction of the external scale is composed of almost pure copper oxide (CuO) followed by an innermost region containing a darker NiCr2O3 spinel layer. Beneath this, there is a deep region containing a mixture of alloy and oxide phases. The islands which have been oxidized are not those of the β and γ phases rich in chromium, but those of the α phase presenting quite small chromium content. The β and γ phase islands are surrounded by a chromia layer close to the alloy/scale interface. Finally, a continuous chromia layer is formed to prevent the alloy against oxidation further.
REFERENCES
[1]Sims C T, Stoloff N S, Hagel W C. Superalloys II[M]. New York: Academic Press, 1987. 153-155.
[2]Kofstad P. High Temperature Corrosion of Metals[M]. New York: Elsevier, 1988. 389-400.
[3]CAO Zhong-qiu, NIU Yan, Gesmundo F. Air oxidation of Cu-50Ni and Cu-70Ni alloys at 800℃[J]. Trans Nonferrous Met Soc China, 2001, 11(4): 499-502.
[4]CAO Zhong-qiu, CAO Li-jie, NIU Yan, et al. Effect of grain size on high-temperature oxidation behavior of Cu-80Ni alloy[J]. Trans Nonferrous Met Soc China, 2003, 13(4): 909-911.
[5]Gesmundo F, Niu Y, Viani F, et al. The transition from the formation of mixed scales to the selective oxidation of the most-reactive component in the corrosion of single and two-phase binary alloys[J]. Oxid Met, 1993, 40: 375-393.
[6]Gesmundo F, Viani F, Niu Y. The possible scaling modes in the high-temperature oxidation of two-phase binary alloys. Part 1: high oxidant pressures[J]. Oxid Met, 1994, 42: 409-429.
[7]Gesmundo F, Viani F, Niu Y, et al. An improvement treatment of the conditions for the exclusive oxidation of the most-reactive component in the corrosion of two-phase binary alloys[J]. Oxid Met, 1994, 42: 465-484.
[8]Gesmundo F, Niu F, Viani F. The possible scaling modes in the high-temperature oxidation of two-phase binary alloys. Part 2: low oxidant pressures[J]. Oxid Met, 1995, 43: 379-394.
[9]Gesmundo F, Gleeson B. Oxidation of multi component two-phase alloys[J]. Oxid Met, 1995, 44: 211.
[10]Gesmundo F, Viani F, Niu Y. The internal oxidation of two-phase binary alloys under low oxidant pressures[J]. Oxid Met, 1996, 45: 51-76.
[11]Smeltzer W, Whittle D P. The criterion for the onset of internal oxidation beneath the external scales[J]. J Electrochem Soc, 1978, 125: 1116-1138.
[12]Gesmundo F, Viani F, Niu Y. The internal oxidation of two-phase binary alloys beneath an external scale of the less-stable oxide[J]. Oxid Met, 1997, 47: 355-380.
[13]Niu Y, Gesmundo F, Wu W T. The air oxidation of two-phase Cu-Ag alloy at 650-750℃[J]. Oxid Met, 1997, 47: 21-52.
[14]Niu Y, Gesmundo F, Douglass D L, et al. The air oxidation of two-phase Cu-Cr alloys at 700-900℃[J]. Oxid Met, 1997, 48; 357-380.
[15]Gesmundo F, Niu Y, Oquab D, et al. The oxidation of two-phase Fe-Cu alloys at 600-800℃[J]. Oxid Met, 1998, 49: 115-146.
[16]Gesmundo F, Niu Y, Viani F, et al. The oxidation of two-phase Cu-Cr alloys under 10-19 atm O2 at 700-900℃[J]. Oxid Met, 1998, 49: 147-167.
[17]Niu F, Li Y S, Gesmundo F. High temperature scaling of two-phase Fe-Cu alloys under low oxygen pressures[J]. Corros Sci, 2000, 42:165-181.
[18]Stott F H, Wood G C, Stringer J. Some limiting factors in the use of alloys at high temperature[J]. Oxid Met, 1995, 44: 113-145.
[19]Villars P, Prince A, Okamoto H. Handbook of Ternary Alloy Phase Diagrams[M]. Ohio: ASM, 1997: 8734.
[20]Cao Z Q, Niu Y, Farne G, et al. Oxidation of the three-phase alloy Cu-20Ni-20Cr at 700-800℃ in 101kPa O2[J]. High Temperature Materials and Processes, 2001, 20: 377-383.
(Edited by YUAN Sai-qian)
Foundation item: Project(50271079) supported by the National Natural Science Foundation of China; Project(202112022) supported by the Education Committee of Liaoning Province
Received date: 2004-06-09; Accepted date: 2005-03-07
Correspondence: CAO Zhong-qiu, Professor, PhD; Tel: +86-24-86593317; E-mail: caozhongqiu6508@sina.com