
Phenomena in late period of seeded precipitation of sodium aluminate solution
LI Xiao-bin(李小斌), FENG Gang-tao(冯港涛), ZHOU Qiu-sheng(周秋生),
PENG Zhi-hong(彭志宏), LIU Gui-hua(刘桂华)
School of Metallurgical Science and Engineering, Central South University, Changsha 410083, China
Received 8 October 2005; accepted 27 February 2006
Abstract: Aiming at seeded precipitation of aluminate solution with high caustic ratio(αk>2.4), corresponding to the late period of seeded precipitation, the influence of different types of seed on precipitation ratio was explained with respect to solution structure in the interface of seed and the evolution of Al(OH)3 growth units in this layer. The effects of solid content and seed size on agglomeration were determined by calculating the particle number of product. The results imply that the solution structure in the interface of seed imposes a notable significance on the process in the late period of seeded precipitation. Agglomeration still exists in this period. However, the agglomeration bodies break in the case of prolonging precipitation due to the mechanical effect, which results in the increase of particle number.
Key words: sodium aluminate solution; seeded precipitation; agglomeration; nucleation; structure
1 Introduction
The seeded precipitation from supersaturated sodium aluminate solution is a crucial step in the Bayer process for the production of alumina, which is a complicated crystallization operation involving a variety of physico-chemistry sub-process such as nucleation, agglomeration and crystal growth. A multitude of researches have been done on the structure and precipitation mechanisms of sodium aluminate solution, but the mechanisms are still obscure and attract considerable research effort[1-4]. So far it is known that the aluminate ion exists in the form of  in moderate concentration and hydrated ion as
in moderate concentration and hydrated ion as 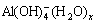 in dilute solution at low temperature, while
in dilute solution at low temperature, while  dehydrates in high concentration solution at high temperature and exists as polymeric ions[5]. The increase of precipitation ratio can be achieved by prolonging the precipitation time, but as generally believed large amounts of secondary nucleation, the breakage of crystals due to mechanical effect and the difficulty of agglomeration because of the low temperature and supersaturation in the late period of seeded precipitation, make the particle size and strength performance of product fall short of the requirements of sandy alumina product[6,7]. Therefore, the further investigation of the late period of seeded precipitation is quite important.
dehydrates in high concentration solution at high temperature and exists as polymeric ions[5]. The increase of precipitation ratio can be achieved by prolonging the precipitation time, but as generally believed large amounts of secondary nucleation, the breakage of crystals due to mechanical effect and the difficulty of agglomeration because of the low temperature and supersaturation in the late period of seeded precipitation, make the particle size and strength performance of product fall short of the requirements of sandy alumina product[6,7]. Therefore, the further investigation of the late period of seeded precipitation is quite important.
2 Experimental
Supersaturated sodium aluminate solutions with caustic ratio αk (the molar ratio of Na2Ok to Al2O3) needed were prepared by dissolving Al (OH) 3(industry grade) into NaOH (industry grade) and then filtered. The solution was poured into stainless steel reactor (volume 2 L) with measure cylinder. After the solution was airproofed and preheated for 10 min, corresponding kinds of seed were added with agitation. At prescribed time, some solutions were sampled and centrifuged. The concentrations of Na2Ok and Al2O3 were determined by volumetry[8] and the particle size distribution of product was analyzed in Mastersizer 2000 apparatus(Malvern Instruments Ltd, UK).
3 Results and discussion
3.1 Effect of seed types on precipitation ratio
In order to investigate the effect of different types of seed on the late period of seeded precipitation, the sodium aluminate solution (αk=1.46 and the concentra-tion of Al2O3 is 195.75 g/L) was precipitated to αk=2.46, then filtered and divided into 5 parts, to which corresponding kinds of seed were added and precipitation continued with constant precipitation temperature 40 ℃. The results are shown in Fig.1. It can be seen from Fig.1 that the process can go on when spent liquid-attached seed (namely filtered but not washed) was added (curve 2). But if the washed seed (curve 1) was added, this process was practically stopped and the same result happened in the case of active seed, such as baked seed[9] and carbonization seed[10] (curve 3-5). The result may relate to the solution structure in the interface of seed surface and the evolution of Al(OH)3 growth units in the interface. According to the Ref.[11],  is the main form containing aluminum in sodium aluminate solution, which can be converted into Al(OH)3 via dehydration, polymerization and the release of OH-.
is the main form containing aluminum in sodium aluminate solution, which can be converted into Al(OH)3 via dehydration, polymerization and the release of OH-.
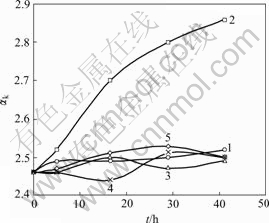
Fig.1 Relationship between precipitation ratio and time with different seeds (initial solid content 600 g/L; solution composition: Na2Ok 181 g/L, αk 2.46; temperature 40 ℃): 1 Washed precipitation seed; 2 Spent liquid-attached precipita- tion seed; 3 Seed dried at 110 ℃ for 2 h; 4 Seed baked at 550 ℃ for 15 min; 5 Washed carbonization seed
When the seeded precipitation is proceeding, the  in spent liquid polymerizes and unites into ion-associated complex
in spent liquid polymerizes and unites into ion-associated complex  based on Eqn.(1):
based on Eqn.(1):
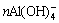 →
→ (1)
(1)
It can be known from the knowledge of industry crystallization that the particles in solution can be formed and precipitated on the surface of seed when the size of the polymer reaches more than that of the critical molecular cluster. Otherwise, it is dissolved[12]. In sodium aluminate solution the size of critical molecular cluster corresponds to the association degree of  . So there exists a critical association degree mcrit which decreases with the increase of supersaturation. The association degree of ion-associated complex
. So there exists a critical association degree mcrit which decreases with the increase of supersaturation. The association degree of ion-associated complex  in the interface of spent liquid-attached seed has reached to some degree. Moreover, it will further increase because the effect of solid force field makes the structure of solute possess strong orientation which results in the augment of
in the interface of spent liquid-attached seed has reached to some degree. Moreover, it will further increase because the effect of solid force field makes the structure of solute possess strong orientation which results in the augment of  concentration and association degree of
concentration and association degree of 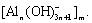 When it exceeds the mcrit, the crystallization of Al(OH)3 happens. This should be why the precipitation can continue when adding the spent liquor-attached seed in the late period of seeded precipitation. As for other kinds of seed, their association degree of
When it exceeds the mcrit, the crystallization of Al(OH)3 happens. This should be why the precipitation can continue when adding the spent liquor-attached seed in the late period of seeded precipitation. As for other kinds of seed, their association degree of  is small in the interface because of the low
is small in the interface because of the low  concentration. It can increase somewhat due to the effect of solid force field. But on the whole, the augment of association degree is quite difficult, especially in the late period of seeded precipitation because of the low supersaturation of solution[13]. Therefore, it is difficult for the association degree in the interface of those kinds of seed to reach the mcrit and the precipitation cannot continue. This result is very significant for the choice of seed in the late period of seeded precipitation.
concentration. It can increase somewhat due to the effect of solid force field. But on the whole, the augment of association degree is quite difficult, especially in the late period of seeded precipitation because of the low supersaturation of solution[13]. Therefore, it is difficult for the association degree in the interface of those kinds of seed to reach the mcrit and the precipitation cannot continue. This result is very significant for the choice of seed in the late period of seeded precipitation.
3.2 Influence of seed size on precipitation ratio and particle number of product in late period of seeded precipitation
The fine and coarse spent liquid-attached seeds were employed to investigate the influence of seed size on the late period of seeded precipitation. The changes of caustic ratio αk with time using different seed size are plotted in Fig.2, which shows that the precipitation ratio using fine seed is obviously much higher than that of coarse seed after 10 h, because the surface area of fine seed is bigger than that of coarse seed, which boosts the precipitation of sodium aluminate solution.
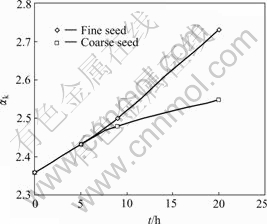
Fig.2 Relationship between precipitation ratio and time with different seed size (initial solid content 600 g/L; solution composition: Na2Ok 171.57 g/L, αk 2.36; temperature 40 ℃)
This paper provides a new criterion concerning agglomeration or nucleation based on the variation of particle numbers and its distribution on different size intervals of seed or product which are calculated by an algorithm[14] (function Calnum.m programmed by MATLAB) pertinent to the PSD patterns and particle population balance method of industry crystallization reactor.
It can be seen from Table 1 that a great number of fine particles disappear in 9 h when adding fine seed. Since the crystal growth rate is very small in the late period of seeded precipitation of sodium aluminate solution because of the low supersaturation (<0.5 μm/d)[15], the losts of so large amount of fine particles may be due to the result of agglomeration. Table 2 shows that agglomeration is more and more obvious with time at the relatively short investigated interval in the experiment with coarse seed. Agglomeration is an automatic process to reduce the surface energy. The finer the seed is, the higher the surface energy is and the stronger the trend that particles automatically agglomerate is. Furthermore, the crystallization of Al(OH)3 prefers to the surface of fine seed for its high activity, which makes the agglomeration body of fine particles firmer because of more binder (the new crystallization of Al(OH)3). Therefore, fine seed can agglomerate more easily. Whereas, the agglomeration of coarse seed needs more binder, so more precipitation duration is needed. It can be broken because of agitation without enough binder. So agglomeration of coarse seeds takes more times than that of fine seeds. In a word, agglomeration still exists in the late period of seeded precipitation of sodium aluminate solution, which causes the mean diameter of product increase.
3.3 Effect of initial solid content on precipitation ratio and characteristic particle number of product
Different contents of spent liquid-attached seed were added in order to determine the effect of solid content on precipitation ratio and the particle number of product on different size intervals in the late period of seeded precipitation. The results are shown in Fig.3, Table 4 and Table 5.
It can be seen from Fig.3 that the precipitation ratio increases with the increase of solid content in the late period of seeded precipitation. As mentioned above, agglomeration exists at relatively short interval in the late period of seeded precipitation of sodium aluminate solution with high αk. But as shown in Table 4 and Table 5 when the experiment is prolonged to 55 h, <20 μm particle number of product is increased by 23.32% with

Fig.3 Relationship between precipitation ratio and time at different solid content (solution composition: Na2Ok 171.57 g/L, αk 2.48; temperature 40 ℃)
Table 1 Variation of characteristic particle numbers of product with fine seed

Table 2 Variation of characteristic particle numbers of product with coarse seed
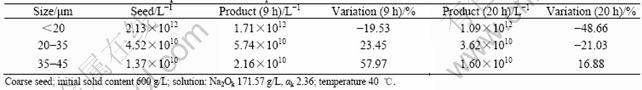
Table 3 Variation of mean size of product with fine and coarse seed

Table 4 Variation of characteristic particle numbers of product with high initial solid content

Table 5 Variation of characteristic particle numbers of product with low initial solid content

high solid content and 12.02% with low solid content. Meanwhile, it can be seen from Table 6 that the mean diameter of product decreases somewhat. It indicates that the agglomeration bodies are not firm and break if agitation time is prolonged in the late period of seeded precipitation of sodium aluminate solution. The increment ratio of particle number of product increases with solid content. The reason is that the more the solid content is in system, the more mechanical collision happens among Al(OH)3 particles.
Table 6 Variation of mean size of product with low and high initial solid content

4 Conclusions
1) The solution structure in the interface of seed affects the process significantly in the late period of seeded precipitation. The precipitation continues only when adding the spent liquid-attached seed.
2) Agglomeration still exists in the late period of seeded precipitation of sodium aluminate solution (αk>2.4).
3) The prolonged precipitation can result in the breakage of agglomeration bodies due to the mechanical effect, which results in the increase of particle number.
References
[1] BI Shi-wen, XUE Hong, YANG Yi-hong, CHEN Wan-kun, WANG Xi-hui, LIU Zhu-zhan, JIANG Xiao-kai. Kinetics of decomposition of Bayer sodium aluminate solution [J]. The Chinese Journal of Nonferrous Metals, 1998, 8(1): 132-134. (in Chinese)
[2] CHEN Qi-yuan, CHEN Guo-hui, YIN Zhou-lan, ZHANG Bin. Nucleation during gibbsites precipitation with seeds from sodium aluminate solution processed under ultrasound [J]. Trans Nonferrous Met Soc China, 2004, 14(2): 401-405.
[3] ZHOU Qiu-sheng, LI Xiao-bin, PENG Zhi-hong, LIU Gui-hua, ZHAO Qing-jie, WU Jie. Kinetics of seeded precipitation from sodium aluminate solution with high concentration [J]. J Cent South Univ (Science and Technology), 2004, 35(4): 557-561. (in Chinese)
[4] CHEN Guo-hui, CHEN Qi-yuan, YIN Zhou-lan, ZHANG Bin. Development of nucleation and agglomeration during decomposition of caustic aluminate solutions with seeds [J]. Hydrometallurgy of China, 2001, 22(1): 14-18. (in Chinese)
[5] XIE Yan-li, BI Shi-wen, YANG Yi-hong, WANG Zhi. Research on structure of sodium aluminate solution in alumina production [J]. Nonferrous Metals, 2001, 53(2): 59-61. (in Chinese)
[6] YANG Zhong-yu. The Technology of Alumina Production [M]. Beijing: Metallurgical Industry Press, 1993. (in Chinese)
[7] BROWN N. A quantitative study of new crystal formation in seeds sodium aluminate solution [J]. J Crystal Growth, 1975, 29: 309-315.
[8] Term of Bayer-Sintering Combination Producted Alumina. Control and Analysis of Bayer-Sintering Combination Producted Alumina [M]. Beijing: Metallurgical Industry Press, 1977.(in Chinese)
[9] SHANGGUAN Zheng. The preparation of active Al(OH)3 seeds [J]. Light Metals, 1995, 8: 12-14. (in Chinese)
[10] WU Yu-sheng, BI Shi-wen, LI Wen-cheng, TONG Zhi-fang, YANG Yi-hong. Effect of carbonization seeds on precipitation of sodium aluminate solution [J]. Light Metals, 2005, 9: 20-23. (in Chinese)
[11] CHEN Qi-yuan. Fundamental Research on Nonferrous Metallurgy—New Methods and Progress[M]. Beijing: Science Press, 2005.(in Chinese)
[12] HIND A R, BHARGAVA S K, GROCOTT S C. The surface chemistry of Bayer process solids: a review [J]. Colloids and Surfaces, 1999, 146: 359-374.
[13] LI Hui-xin, ADDAI-MENSAH J, THOMAS J C, GERSON A R. The crystallization mechanism of Al(OH)3 from sodium aluminate solutions [J]. Journal of Crystal Growth, 2005, 279: 508-520.
[14] XU Xiao-hui. Study on the Model of Crystal Growth During Seeded Precipitation of Sodium Aluminate Solution [D]. Changsha: Central South University, 2004. (in Chinese)
[15] VEESLER S, ROURCE S, BOISTELLE R. About supersaturation and growth rates of hydragillite Al(OH)3 in alumina sodium solution [J]. J Crystal Growth, 1993, 130: 411-415.
(Edited by YUAN Sai-qian)
Foundation item: Project(2005CB623702) supported by the National Basic Research Program of China
Corresponding author: LI Xiao-bin; Tel: +86-731-8877830; E-mail: X.B.Li@mail.csu.edu.cn