高岭石与氧化铁在还原焙烧过程中的反应行为
来源期刊:中国有色金属学报(英文版)2019年第1期
论文作者:李小斌 王洪阳 周秋生 齐天贵 刘桂华 彭志宏 王一霖
文章页码:186 - 193
关键词:高岭土;氧化铁;铝酸亚铁;石英固溶体;方石英固溶体;还原焙烧
Key words:kaolin; Fe2O3; hercynite; quartz solid solution; cristobalite solid solution; reduction roasting
摘 要:铝硅酸盐矿物中氧化硅和氧化铝的预先分离对从高硅含铝物料中提取氧化铝具有重要意义。本文作者 研究高岭石与氧化铁在还原焙烧过程中的反应行为。热力学计算以及还原焙烧实验结果表明,由氧化铁还原得到的氧化亚铁优先与高岭石中的氧化铝反应而生成铝酸亚铁;与此同时,高岭石中的氧化硅转变为石英固溶体或者方石英固溶体。在有足量碳粉存在的条件下,过量的氧化亚铁和氧化硅反应生成的硅酸亚铁随着焙烧温度的升高进一步还原成氧化硅和金属铁。然而,升高焙烧温度及降低Fe2O3/Al2O3的摩尔比均能促进莫来石的形成。控制高岭土、赤铁矿和煤灰中Fe2O3/Al2O3/C摩尔比为1.2:2.0:1.2,在1373 K下还原焙烧60 min即可实现高岭石完全转变为独立的氧化硅和铝酸亚铁。研究结果有望为铝硅酸盐矿物中氧化铝和氧化硅的综合利用提供新技术。
Abstract: The pre-separation of silica and alumina in aluminosilicates is of great significance for efficiently treating alumina-/ silica-bearing minerals for alumina production. In this work, the reaction behavior of kaolinite with ferric oxide during reduction roasting was investigated. The results of thermodynamic analyses and reduction roasting experiments show that ferrous oxide obtained from ferric oxide reduction preferentially reacts with alumina in kaolinite to form hercynite, meanwhile the silica in kaolinite is transformed into quartz solid solution and/or cristobalite solid solution. With increasing roasting temperature, fayalite formed by reaction of surplus ferrous oxide with silica at low temperature is reduced to silica and metallic iron in the presence of sufficient carbon dosage. However, increasing roasting temperature and decreasing Fe2O3/Al2O3 molar ratio favor mullite formation. The complete conversion of kaolinte into free silica and hercynite can be obtained by roasting raw meal of kaolin, ferric oxide and coal powder with Fe2O3/Al2O3/C molar ratio of 1.2:2.0:1.2 at 1373 K for 60 min. This work may facilitate the development of a technique for comprehensively utilizing silica and alumina in aluminosilicates.
Trans. Nonferrous Met. Soc. China 29(2019) 186-193
Xiao-bin LI, Hong-yang WANG, Qiu-sheng ZHOU, Tian-gui QI, Gui-hua LIU, Zhi-hong PENG, Yi-lin WANG
School of Metallurgy and Environment, Central South University, Changsha 410083, China
Received 7 December 2017; accepted 16 May 2018
Abstract: The pre-separation of silica and alumina in aluminosilicates is of great significance for efficiently treating alumina-/ silica-bearing minerals for alumina production. In this work, the reaction behavior of kaolinite with ferric oxide during reduction roasting was investigated. The results of thermodynamic analyses and reduction roasting experiments show that ferrous oxide obtained from ferric oxide reduction preferentially reacts with alumina in kaolinite to form hercynite, meanwhile the silica in kaolinite is transformed into quartz solid solution and/or cristobalite solid solution. With increasing roasting temperature, fayalite formed by reaction of surplus ferrous oxide with silica at low temperature is reduced to silica and metallic iron in the presence of sufficient carbon dosage. However, increasing roasting temperature and decreasing Fe2O3/Al2O3 molar ratio favor mullite formation. The complete conversion of kaolinte into free silica and hercynite can be obtained by roasting raw meal of kaolin, ferric oxide and coal powder with Fe2O3/Al2O3/C molar ratio of 1.2:2.0:1.2 at 1373 K for 60 min. This work may facilitate the development of a technique for comprehensively utilizing silica and alumina in aluminosilicates.
Key words: kaolin; Fe2O3; hercynite; quartz solid solution; cristobalite solid solution; reduction roasting
1 Introduction
Aluminosilicates, which commonly exist in some natural mineral resources as impurities and decrease the grade of valuable minerals, are removed to tailing during handling the raw ore. Typically, the production of 1 t bauxite concentrate with A/S (mass ratio of alumina to silica) of 8-10 will generate about 0.3 t bauxite tailing with A/S of 1.5-2.0 by treating raw bauxite with A/S of 5-6 [1,2]. In 2016, the bauxite output was about 6.5×107 t in China [3], and most of the bauxite tailings are deposited on land surface [4]. Besides, more than 5×109 t of coal gangue, which is a type of kaolinite-rich material and produced by coal mining and washing, is stockpiled owing to the low utilization in China [5], and the amount increases by (1.5-2.0)×108 t annually [6]. The major chemical compositions are alumina and silica in both bauxite tailing and coal gangue, while major mineralogical compositions in bauxite tailing are diaspore and aluminosilicates, and those in coal gangue are aluminosilicates. Thus, the comprehensive extraction of alumina and silica in the aluminosilicates is the key to realize large-scale and high-value applications of bauxite tailing and coal gangue.
In recent decades, much attention has been paid to alumina extraction from aluminosilicates, which can be divided into acid process and alkali process according to the leaching reagents. The former focused on alumina extraction through leaching aluminosilicates treated by mechanical activation [6], thermal activation [7] or sintering with Na2CO3 [8], using sulfuric acid or hydrochloric acid as leaching agent; however, this process generated a large amount of wastewater, required corrosion-resistant equipment and is quite complicated due to iron removal. Whereas the latter mainly aimed at alumina extraction through alkali leaching of aluminosilicates treated by sintering with Na2CO3- CaCO3 [9] or silica removal through alkali leaching of aluminosilicates treated by thermal activation [10-12] in order to offer qualified raw materials for the Bayer alumina refinery. In fact, soda-lime sintering process is not able to treat aluminosilicates for its high energy consumption and inability to utilize silica. So, roasting- leaching process is regarded as a potential way to handle aluminosilicates for comprehensive utilization of silica and alumina [13].
In the roasting process, for instance, kaolinite dehydrates and turns into metakaolinite at about 823 K, then the metakaolinite splits into free SiO2 (amorphous), free Al2O3 (amorphous or gamma phase) and even Al-Si spinel at 1253 K [14-19]. In fact, there are disputes in Al-Si spinel form, i.e. SiO2·6Al2O3 or 3Al2O3·2SiO2 [14,15,19], while the occurrence of free Al2O3 is even doubtful due to lack of enough evidences. Because the A/S of leaching residue is generally less than that of mullite [14], it seems that most of the alumina in roasted product exists in 3Al2O3·2SiO2 Al-Si spinel, resulting in difficulty in deeply separating silica and alumina by following alkali leaching. Completely converting kaolinite into a silica-free aluminum compound and free SiO2 in roasting might favor separation between silica and alumina, provided that the aluminum compound is inactive in the subsequent leaching process. Studies showed that kaolinite could react with some additives in roasting process, such as MgO, FeO, ZnO and CuO, forming corresponding alumina spinel and quartz solid solution [20,21], but there still exists either fayalite or mullite in roasted product. Thus, in view of hematite in bauxite, the reaction behavior for the system Fe2O3-SiO2-Al2O3 in reduction roasting was studied by using pure compounds as raw materials in our previous work [22]. Unfortunately, no enough attention was paid to aluminosilicates, especially to transformation mechanism of aluminosilicates in roasting process.
Given that the kaolinite is a typical aluminosilicate in bauxite and its reaction behavior in roasting is similar to other aluminosilicates such as illite and pyrophyllite, kaolin was employed in this work. Based on thermodynamic analysis for reactions in roasting the mixed raw meals of kaolin, ferric oxide and coal powder, phase transformations in reduction roasting of kaolin and ferric oxide were investigated by XRD and SEM.
2 Experimental
2.1 Materials
Fe2O3 (d50=2.80 μm) used in this work is of analytical grade, while coal powder used as reductant has a fixed carbon content of 76.47 wt.%. The chemical composition and XRD patterns of kaolin (d50=2.82 μm) are shown in Table 1 and Fig. 1, respectively. As shown in Fig. 1, kaolin mainly consists of kaolinite and a small amount of quartz. Besides, the quartz content in kaolin is about 1.46 wt.% calculated by A/S.
Table 1 Chemical composition of kaolin (wt.%)

2.2 Experimental procedure
2.2.1 Raw meal preparation
In order to obtain well-mixed raw meals, the raw meals of kaolin, ferric oxide and coal powder were mixed by vibrating mill for 2 min. It should be noted that the amount of coal powder was determined by stoichiometric proportion for converting ferric oxide to ferrous oxide based on the fixed carbon changing to carbon monoxide.
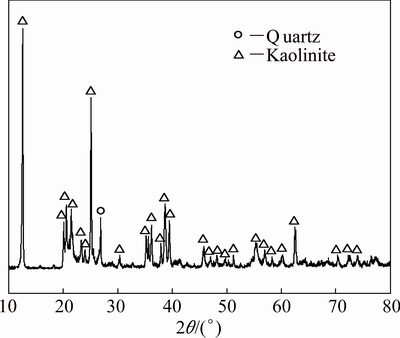
Fig. 1 XRD pattern of kaolin
2.2.2 Reduction roasting
10 g mixed raw meal was placed into a 30 mL corundum crucible covered by a lid with a layer of coke powder on it, and then a small corundum crucible was put in a 100 mL corundum crucible with a layer of powdered coke on the inner lining and the bottom. The big corundum crucible was also covered by a lid in order to guarantee the reductive atmosphere during the roasting process. Subsequently, the big corundum crucible was placed and heated in a thermostatic muffle furnace at preset temperatures for certain duration, and then taken out from the furnace and cooled in air to room temperature. The cooled reduction roasting product was namely clinker.
2.3 Analysis methods
The phase analyses of clinkers were performed on powder using a X-ray diffractometer (TTR-III, Rigaku Corporation, Japan) with Cu Kα radiation (λ=1.5406 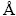 ). Data were recorded at 2θ from 10° to 80° and a step size of 0.02° was used at a scan rate of 10 (°)/min. Surface microscopic morphology and micro area composition analyses were conducted by SEM (JSM-6360LV, JEOL, Japan) and EDX (GENSIS60S, EDAX, USA) respectively. The polished clinker samples were prepared by mixing clinker with both epoxy resin and triethanolamine followed by polishing.
). Data were recorded at 2θ from 10° to 80° and a step size of 0.02° was used at a scan rate of 10 (°)/min. Surface microscopic morphology and micro area composition analyses were conducted by SEM (JSM-6360LV, JEOL, Japan) and EDX (GENSIS60S, EDAX, USA) respectively. The polished clinker samples were prepared by mixing clinker with both epoxy resin and triethanolamine followed by polishing.
3 Results and discussion
3.1 Thermodynamic analysis on reactions of kaolinite with Fe2O3 during reduction roasting
As mentioned above, fayalite and mullite were formed more or less in reduction roasting of kaolin mixing with ferric oxide or iron (II) oxalate [20,21]. In order to avoid forming these poly-compounds containing silica, it is meaningful to carry out thermodynamic analysis on reactions during reduction roasting of kaolinite with Fe2O3.
The possible reactions were listed in Eqs. (1)-(6) between kaolinite and Fe2O3 with carbon as reductive agent, while those were described in Eqs. (7)-(11) when roasting kaolinite alone. In convenience of comparison, the stoichiometric coefficient of SiO2 was normalized to be 2.
Al2O3·2SiO2·2H2O+0.5Fe2O3+0.5C=FeAl2O4+2SiO2+0.5CO(g)+2H2O(g) (1)
Al2O3·2SiO2·2H2O+2.5Fe2O3+2.5C=FeAl2O4+2(2FeO·SiO2)+2.5CO(g)+2H2O(g) (2)
3FeAl2O4+2SiO2+3C=3Al2O3·2SiO2+3Fe+3CO(g) (3)
4FeAl2O4+2SiO2=2(2FeO·SiO2)+4Al2O3 (4)
2Fe2O3+2SiO2+2C=2(2FeO·SiO2)+2CO(g) (5)
2(2FeO·SiO2)+2C=4Fe+2SiO2+2CO(g) (6)
Al2O3·2SiO2·2H2O=1/3(3Al2O3·2SiO2)+4/3SiO2+2H2O(g) (7)
Al2O3·2SiO2·2H2O=Al2O3+2SiO2+2H2O(g) (8)
Al2O3·2SiO2=1/3(3Al2O3·2SiO2)+4/3SiO2 (9)
Al2O3·2SiO2=Al2O3+2SiO2 (10)
3Al2O3+2SiO2=3Al2O3·2SiO2 (11)
The calculated relationships between standard Gibbs free energy change 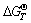 and temperature T were plotted for the reactions between kaolinite and Fe2O3 in presence of carbon in Fig. 2, in which the thermodynamic data were derived from literature [23] and the calculations were based on the data of quartz and γ-Al2O3 for SiO2 and Al2O3. Thermodynamic data of kaolinite at 700-1600 K were as △Gf=1.07T-4119.53 and R2=1.00 calculated by linear equation, which were extrapolated according to the relevant data at 298.15-700 K [23].
and temperature T were plotted for the reactions between kaolinite and Fe2O3 in presence of carbon in Fig. 2, in which the thermodynamic data were derived from literature [23] and the calculations were based on the data of quartz and γ-Al2O3 for SiO2 and Al2O3. Thermodynamic data of kaolinite at 700-1600 K were as △Gf=1.07T-4119.53 and R2=1.00 calculated by linear equation, which were extrapolated according to the relevant data at 298.15-700 K [23].
From Fig. 2(a), it can be known that the conversion of kaolinite to mullite can occur readily in roasting process because of the small 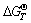 for Reaction (7), but Reaction (1) is likely to proceed prior to Reaction (7) thermodynamically. Thus, reduction roasting of kaolinite in the presence of Fe2O3 generates hercynite and may prevent kaolinite from forming mullite. The hercynite might be spontaneously formed either through Reaction (1) for low Fe2O3/Al2O3 molar ratio or Reaction (2) for high Fe2O3/Al2O3 molar ratio, but the former reaction can occur more readily than the latter thermodynamically due to its more negative value of
for Reaction (7), but Reaction (1) is likely to proceed prior to Reaction (7) thermodynamically. Thus, reduction roasting of kaolinite in the presence of Fe2O3 generates hercynite and may prevent kaolinite from forming mullite. The hercynite might be spontaneously formed either through Reaction (1) for low Fe2O3/Al2O3 molar ratio or Reaction (2) for high Fe2O3/Al2O3 molar ratio, but the former reaction can occur more readily than the latter thermodynamically due to its more negative value of 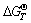 . That is to say, fayalite formation can be avoided by kaolinite reacting with Fe2O3 (Reaction (2)) in the case of theoretical Fe2O3 dosage according to Reaction (1), while the transformation of kaolinite to hercynite can be completed. Moreover, mullite can be formed by Reaction (3) when the temperature exceeds 1288 K, suggesting that relatively low temperature is required in reduction roasting process to prevent the hercynite from further converting to mullite.
. That is to say, fayalite formation can be avoided by kaolinite reacting with Fe2O3 (Reaction (2)) in the case of theoretical Fe2O3 dosage according to Reaction (1), while the transformation of kaolinite to hercynite can be completed. Moreover, mullite can be formed by Reaction (3) when the temperature exceeds 1288 K, suggesting that relatively low temperature is required in reduction roasting process to prevent the hercynite from further converting to mullite.
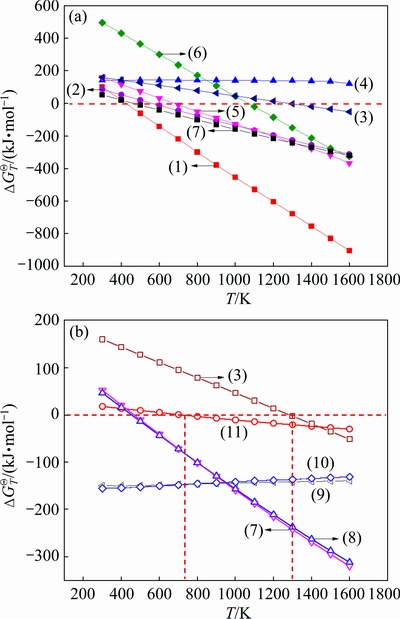
Fig. 2 Relationships between 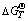 and T for Eqs. (1)-(11)
and T for Eqs. (1)-(11)
Although hercynite and free silica generated by Reaction (1) are unable to react to form fayalite according to Reaction (4) due to the positive 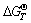 , the fayalite can be produced by reaction of Fe2O3 with free silica through Reaction (5), meaning that excessive Fe2O3 dosage is conducive to fayalite formation. Considering appropriately excessive Fe2O3 dosage being inevitable for completing the solid-state conversion of kaolinite to hercynite, the fayalite will be generated unavoidably and can be further converted to metallic iron and free silica by Reaction (6) in the presence of sufficient carbon dosage.
, the fayalite can be produced by reaction of Fe2O3 with free silica through Reaction (5), meaning that excessive Fe2O3 dosage is conducive to fayalite formation. Considering appropriately excessive Fe2O3 dosage being inevitable for completing the solid-state conversion of kaolinite to hercynite, the fayalite will be generated unavoidably and can be further converted to metallic iron and free silica by Reaction (6) in the presence of sufficient carbon dosage.
In order to better understand the mullite formation, the possible reactions in roasting kaolinite are thermodynamically analyzed and the results are drawn in Fig. 2(b), in which the influence of T on 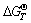 for Reaction (3) is also shown to enable a visual comparison. Figure 2(b) obviously shows that the decomposition of kaolinite can occur thermodynamically through either Reaction (7) or Reaction (8), forming free silica accompanying mullite or free alumina respectively. In fact, metakaolinite might be generated preferentially due to dehydration of kaolinite and consequently decomposes according to Reactions (9) and (10). It should be noted that, the difference of
for Reaction (3) is also shown to enable a visual comparison. Figure 2(b) obviously shows that the decomposition of kaolinite can occur thermodynamically through either Reaction (7) or Reaction (8), forming free silica accompanying mullite or free alumina respectively. In fact, metakaolinite might be generated preferentially due to dehydration of kaolinite and consequently decomposes according to Reactions (9) and (10). It should be noted that, the difference of 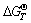 at any calculation temperature is very small either between Reactions (7) and (8) or between Reactions (9) and (10), the
at any calculation temperature is very small either between Reactions (7) and (8) or between Reactions (9) and (10), the 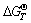 of reactions for forming mullite (Reactions (7) and (9)) has even a little smaller value than that for forming free alumina (Reactions (8) and (10)) when roasting temperature exceeds 767 K. Meanwhile, even if free alumina and free silica are generated, they would react further to form mullite through Reaction (11) at above 760 K. Hence, mullite formation is also unavoidable thermodynamically in roasting kaolinite alone even at very low temperature. Because higher temperature is required for mullite formation in reduction roasting process (Reaction (3)) than that in roasting kaolinite alone, the reduction roasting process favors avoiding mullite formation and thus converting the silica in kaolinite into free silica as much as possible.
of reactions for forming mullite (Reactions (7) and (9)) has even a little smaller value than that for forming free alumina (Reactions (8) and (10)) when roasting temperature exceeds 767 K. Meanwhile, even if free alumina and free silica are generated, they would react further to form mullite through Reaction (11) at above 760 K. Hence, mullite formation is also unavoidable thermodynamically in roasting kaolinite alone even at very low temperature. Because higher temperature is required for mullite formation in reduction roasting process (Reaction (3)) than that in roasting kaolinite alone, the reduction roasting process favors avoiding mullite formation and thus converting the silica in kaolinite into free silica as much as possible.
In one word, thermodynamically, the formation of both mullite and fayalite could be avoided by reduction roasting process instead of roasting kaolinite alone, through calibrating ingredients in raw meal including kaolinite, ferric oxide and carbon as well as adjusting appropriate roasting temperature. In order to verify the thermodynamic results, the corresponding reduction roasting experiments were conducted.
3.2 Reactions of raw meal of kaolin and ferric oxide in reduction roasting process
3.2.1 Effect of roasting time
Firstly, the influence of roasting time on reactions of kaolin and ferric oxide with coal powder as reductive agent was investigated experimentally. Raw meal was prepared by mixing kaolin, ferric oxide and coal powder with Fe2O3/Al2O3/C molar ratio of 1.0:2.0:1.0, and then roasted at 1373 K for 30, 60 and 120 min, respectively. XRD patterns of the obtained clinkers are presented in Fig. 3. The XRD pattern of the clinker for 30 min indicates that the quartz solid solution and hercynite emerge in the clinker, accompanying the formation of a little metallic iron shown in the XRD pattern of partial enlargement. When the raw meals are roasted at 1373 K for longer than 60 min, the typical diffraction peaks of both cristobalite solid solution and mullite appear in the XRD pattern of clinkers, meanwhile the diffraction apices are enhanced with increasing roasting time. In addition, the diffraction apex of metallic iron at 2θ of 44.8° increases significantly with increasing roasting time, e.g. the intensities are 65, 119 and 212 for roasting time of 30, 60 and 120 min, respectively. The results mentioned above indicate that hercynite can be readily formed by Reaction (1) and consequently react with free silica to generate mullite and metallic iron by Reaction (3) under this condition, which coincides with the results of the previous thermodynamic analyses.
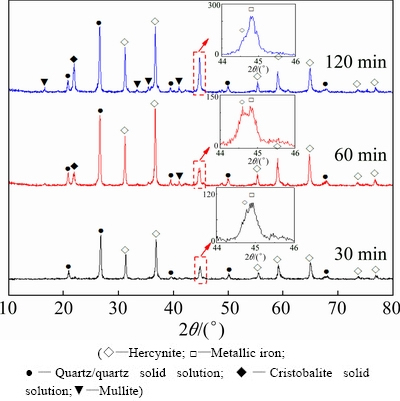
Fig. 3 XRD patterns of clinkers prepared by roasting raw meals at 1373 K for different time
Besides, the weak diffraction peaks of hercynite and quartz solid solution with duration of 30 min mean that amorphous phases still exist in the clinker, which suggests that the phase conversions do not proceed completely. Meanwhile, accompanied by the decrease of hercynite diffraction apices, the diffraction apices of mullite in the clinker by roasting raw meal for 120 min are obviously stronger than those for 60 min. Hence, roasting time of 60 min is appropriate to fully convert the silica in kaolinite into free state.
Moreover, the characteristic diffraction peaks observed in the XRD patterns of clinkers are similar to those of quartz and cristobalite. The quartz-type structure was defined as quartz solid solution [20] for the entrance of Fe2+ into the open spaces of silicon oxygen tetrahedra caused by replacement of Si4+ by Al3+ to render the structure electrically neutral, while the cristobalite-type structure can be similarly defined as cristobalite solid solution. In addition, both quartz solid solution and cristobalite solid solution are readily soluble in dilute alkali solution under atmospheric pressure, which is quite different from that of quartz and cristobalite. It should also be noted that the characteristic diffraction peaks of quartz in kaolin (Fig. 1) and quartz solid solution in clinkers substantially overlap at 2θ of 26.6°. Considering the relatively low content of quartz in kaolin, it is believed that the peak is mainly contributed by quartz solid solution. Besides, amorphous silica might exist in clinkers according to the researches on roasting kaolin alone [14,16,17].
3.2.2 Effect of Fe2O3/Al2O3 molar ratio
As Fe2O3 dosage has an important effect not only on the phase conversion of raw meal in reduction roasting process but also on the economics of pre-treating high silica bauxite to produce alumina, the effect of Fe2O3/Al2O3 molar ratio on the phase conversion was examined. Figure 4 shows the XRD patterns of the clinkers prepared by roasting raw meals at 1223 and 1373 K for 60 min with different Fe2O3/Al2O3 molar ratios. From Fig. 4, it can be known that the formation of mullite strongly depends on Fe2O3/Al2O3 molar ratio in raw meal, i.e. low Fe2O3/Al2O3 molar ratio favors the formation of mullite. The XRD pattern indicates that mullite even exists in the clinker by roasting the raw meal at 1373 K for 60 min with Fe2O3/Al2O3 molar ratio of 1.0:2.0, while the silica in the clinker presents in the form of quartz solid solution and cristobalite solid solution. When Fe2O3/Al2O3 molar ratio is above 1.2:2.0, the characteristic diffraction peaks of both mullite and quartz solid solution cannot be observed in the XRD pattern of clinkers, meanwhile the diffraction apices of hercynite and cristobalite solid solution increase significantly with increasing Fe2O3/Al2O3 molar ratio. In addition, the metallic iron diffraction apexes in the XRD patterns of clinkers increase consistently with increasing Fe2O3/Al2O3 molar ratio. Figure 4 also shows that fayalite can be detected only in the clinkers prepared by roasting high Fe2O3/Al2O3 molar ratio (≥1.4:2.0) raw meal at relatively low temperature (1223 K), otherwise fayalite cannot be found in the clinkers under other conditions. This variation tendency of fayalite can be explained by reduction of fayalite to silica and metallic iron by Reaction (6) as discussed in Section 3.1, although there exist differences in temperature and Fe2O3/Al2O3 molar ratio between thermodynamic analyses and experiments.
Therefore, considering the existence of mullite in the clinker prepared by roasting raw meal with theoretical Fe2O3/Al2O3 molar ratio of 1.0:2.0, the Fe2O3/Al2O3 molar ratio of 1.2:2.0 is appropriate in order to guarantee both the minimum Fe2O3 dosage and complete conversion of silica in kaolinite into free silica.
3.2.3 Effect of roasting temperature
As roasting temperature has a marked impact on phase conversion of raw meal during reduction roasting as discussed in Section 3.1, the effect of temperature on roasting reactions was experimentally investigated in detail. XRD patterns are presented in Fig. 5 for the clinkers prepared by roasting raw meals with various Fe2O3/Al2O3 molar ratios at different roasting temperatures.
When roasting the raw meals at relatively low temperature (1173 K), mullite can be observed in all clinkers by XRD analysis, but its characteristic diffraction peaks fade with increasing Fe2O3/Al2O3 molar ratio. By treating raw meal with Fe2O3/Al2O3 molar ratio ≤1.0:2.0, it can be seen by comparing Figs. 5(a) and (b) that XRD peaks of mullite in clinkers become more prominent with increasing roasting temperature, implying more perfect crystallization morphology; whereas, by treating raw meal with Fe2O3/Al2O3 molar ratios of 1.2:2.0 and 1.4:2.0, diffraction peaks of mullite disappear in XRD patterns of clinkers obtained at 1273 and 1373 K, respectively, while they emerge again at 1423 and 1473 K correspondingly. As for enough Fe2O3 dosage, hercynite is the most stable phase thermodynamically according to Reaction (1), but the local inhomogeneous composition might cause direct decomposition of kaolinite to form mullite at low temperatures (<1273 K) through Reaction (7). Moreover, the reaction of hercynite and silica through Reaction (3) results in the mullite formation at temperatures higher than 1423 K.
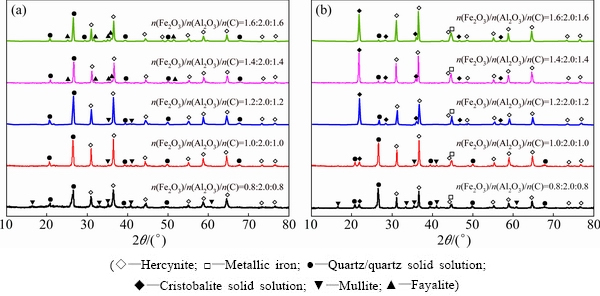
Fig. 4 XRD patterns of clinkers prepared by roasting raw meals with different Fe2O3/Al2O3/C molar ratios at 1223 K (a) and 1373 K (b) for 60 min
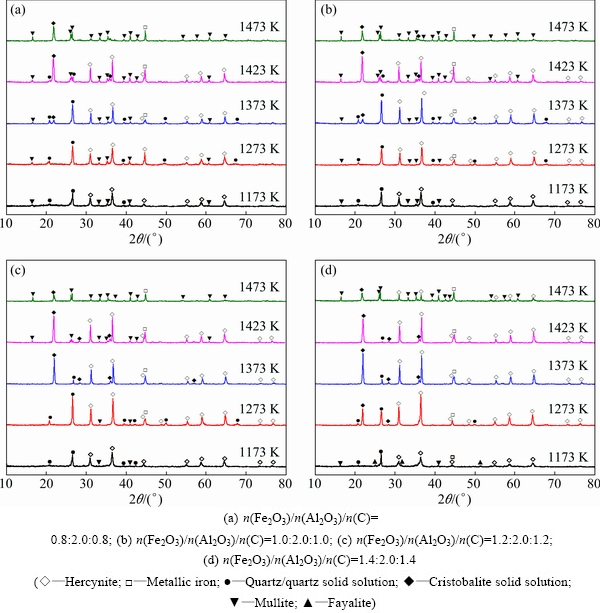
Fig. 5 XRD patterns of clinkers prepared by roasting raw meals at different temperatures for 60 min
The existing forms of silica in clinkers are affected not only by Fe2O3/Al2O3 molar ratio as mentioned in Section 3.2.2 but also by roasting temperature. Generally, increasing roasting temperature (1173-1423 K) can facilitate the conversion of quartz solid solution to cristobalite solid solution, represented by the decrease in diffraction apex at 2θ of 26.6° for quartz solid solution accompanying increase in that at 2θ of 22.0° for cristobalite solid solution. In addition, it is also deduced from Fig. 5 that the increase of Fe2O3/Al2O3 molar ratio is beneficial to reducing the conversion temperature. However, the diffraction apices of cristobalite solid solution and hercynite remarkably decrease in the XRD patterns of clinkers obtained at 1473 K, which can be attributed to the reaction of hercynite and cristobalite solid solution to form mullite by Reaction (3).
Hercynite diffraction apex at 2θ of 36.5°, as shown in Fig. 5, is enhanced significantly with increasing roasting temperature from 1173 to 1423 K, indicating that raising roasting temperature can promote hercynite formation. Furthermore, it can be considered that excessive Fe2O3 dosage can hinder the transformation of hercynite to mullite at 1473 K by comparing Fig. 5(d) with Figs. 5(a-c). This result is ascribed to the fact that the hercynite particles are coarsened under the connection effect of metallic iron phase formed at high temperatures by reduction of iron-containing compounds and thus impede hercynite reacting with cristobalite solid solution, as shown in Fig. 6.
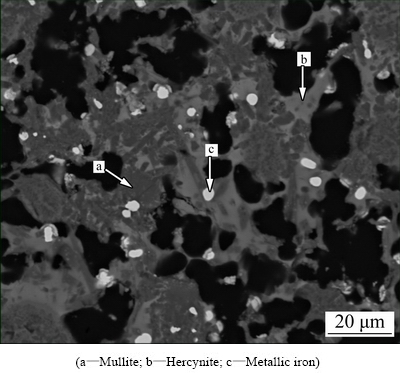
Fig. 6 SEM image of clinker prepared by roasting raw meal with Fe2O3/Al2O3/C molar ratio of 1.4:2.0:1.4 at 1473 K for 60 min
In summary, adopting proper roasting temperature and Fe2O3/Al2O3 molar ratio in raw meal can achieve complete conversion of kaolinte into free silica and hercynite, the optimal condition can be described as Fe2O3/Al2O3/C molar ratio of 1.2:2:1.2, roasting temperature of 1373 K and roasting duration of 60 min.
4 Conclusions
(1) Thermodynamic analyses and experiments show that in reduction roasting of the mixture of kaolin, ferric oxide and carbon, raising temperature favors the conversion of kaolinite to hercynite and free silica, whereas excessively high temperature and insufficient Fe2O3 dosage benefit mullite formation.
(2) The free silica in clinkers includes quartz solid solution and cristabolite solid solution, and the increase of both roasting temperature and Fe2O3/Al2O3 molar ratio facilitates quartz solid solution transforming to cristabolite solid solution.
(3) The complete conversion of kaolinite into free silica and hercynite can be achieved under the following optimal conditions: Fe2O3/Al2O3/C molar ratio of 1.2:2:1.2, roasting temperature of 1373 K, roasting duration of 60 min.
References
[1] WANG Y, HU Y, HE P, GU G. Reverse flotation for removal of silicates from diasporic-bauxite [J]. Minerals Engineering, 2004, 17: 63-68.
[2] XIA Liu-yin, ZHONG Hong, LIU Guang-yi, HUANG Zhi-qiang, CHANG Qing-wei. Flotation separation of the aluminosilicates from diaspore by a Gemini cationic collector [J]. International Journal of MineralProcessing, 2009, 92: 74-83.
[3] USGS (United States Geological Survey). Mineral commodity summaries: Bauxite and alumina [EB/OL]. https://minerals.usgs.gov/ minerals/pubs/ commodity/bauxite/mcs-2017-bauxi.pdf
[4] LIU Hai-bin, LIU Zhen-ling. Recycling utilization patterns of coal mining waste in China [J]. Resources, Conservation and Recycling, 2010, 54: 1331-1340.
[5] GUO Yan-xia, LV Hui-bin, YANG Xi, CHENG Fang-qin. AlCl3·6H2O recovery from the acid leaching liquor of coal gangue by using concentrated hydrochloric inpouring [J]. Separation and Purification Technology, 2015, 151: 177-183.
[6] XIAO Jin, LI Fa-chuang, ZHONG Qi-fan, BAO Hong-guang, WANG Bing-jie, HUANG Jin-di, ZHANG Yan-bing. Separation of aluminum and silica from coal gangue by elevated temperature acid leaching for the preparation of alumina and SiC [J]. Hydrometallurgy, 2015, 155: 118-124.
[7] LI Guang-hui, ZENG Jing-hua, LUO Jun, LIU Ming-xia, JIANG Tao, QIU Guan-zhou. Thermal transformation of pyrophyllite and alkali dissolution behavior of silicon [J]. Applied Clay Science, 2014, 99: 282-288.
[8] YAN Ke-zhou, GUO Yan-xia, FANG Li, CUI Li, CHENG Fang-qin, LI Tong-yang. Decomposition and phase transformation mechanism of kaolinite calcined with sodium carbonate [J]. Applied Clay Science, 2017, 147: 90-96.
[9] GENG Xue-wen, MA Hong-wen, SU Shuang-qing, MA Shi-lin. The preparation of aluminum hydroxide from high-alumina gangue desilication residues based on soda lime sintering method [J]. Bulletin of Mineralogy, Petrology and Geochemistry, 2012, 31(6): 635-639. (in Chinese)
[10] QIU Guan-zhou, JIANG Tao, LI Guang-hui, FAN Xiao-hui, HUANG Zhu-cheng. Activation and removal of silicon in kaolinite by thermochemical process [J]. Scandinavian Journal of Metallurgy, 2004, 33: 121-128.
[11] BONN H S, GINSBERG H. Method for reducing the silica content of alumina-containing materials of the clay type: US patent, 2939764 [P]. 1960-06-07.
[12] RAYZMAN V L, PEVZNER I Z, SIZYAKOV V M, NI L P, FILIPOVICH I K, ATURIN A V.Extracting silica and alumina from low-grade bauxite [J]. Journal of the Minerals, Metals & Materials Society, 2003, 55: 47-50.
[13] SMITH P. The processing of high silica bauxites—Review of existing and potential processes [J]. Hydrometallurgy, 2009, 98: 162-176.
[14] CHAKRABORTY A K, GHOSH D K. Reexamination of the kaolinite-to-mullite reaction series [J]. Journal of the American Ceramic Society, 1978, 61(3-4): 170-173.
[15] CHAKRABORTY A K. Formation of silicon-aluminum spinel [J]. Journal of the American Ceramic Society,1979, 62(3-4): 120-124.
[16] LEE S, KIM Y J, MOON H S. Phase transformation sequence from kaolinite to mullite investigated by an energy-filtering transmission electron microscope [J]. Journal of the American Ceramic Society, 1999, 82(11): 2841-2848.
[17] OKADA K, OTSUKA N, OSSAKA J. Characterization of spinel phase formed in the kaolinite-mullite thermal sequence [J]. Journal of the American Ceramic Society, 1986, 69(10): C251-C253.
[18] SANZ J, MADANI A, SERRATOSA J M. Aluminum-27 and silicon-29 magic-angle spinning nuclear magnetic resonance study of the kaolinite-mullite transformation [J]. Journal of the American Ceramic Society, 1988, 71(10): C418-C421.
[19] SONUPARLAK B, SARIKAYA M, AKSAY I A. Spinel phase formation during the 980 °C exothermic reaction in the kaolinite-to- mullite reaction series [J]. Journal of the American Ceramic Society, 1987, 70(11): 837-842.
[20] SEGNIT E R, GELB T. Metastable quartz-type structures formed from kaolinite by solid state reaction [J]. American Mineralogist, 1972, 57: 1505-1514.
[21] TAKEUCHI N, TAKAHASHI H, ISHIDA S, HORIIE F, WAKAMATSU M. Mechanistic study of solid-state reaction between kaolinite and ferrous oxide at high temperatures [J]. Journal of the Ceramic Society of Japan, 2000, 108(10): 876-881.
[22] ZHOU Qiu-sheng, LI Chuang, LI Xiao-bin, PENG Zhi-hong, LIU Gui-hua, QI Tian-gui. Reaction behavior of ferric oxide in system Fe2O3-SiO2-Al2O3 during reductive sintering process [J]. Transactions of Nonferrous Metals Society of China, 2016, 26: 842-848.
[23] BARIN I. Thermochemical data of pure substances [M]. Weinheim: VCH Verlagsgesellschaft mbH, 1995.
李小斌,王洪阳,周秋生,齐天贵,刘桂华,彭志宏,王一霖
中南大学 冶金与环境学院,长沙 410083
摘 要:铝硅酸盐矿物中氧化硅和氧化铝的预先分离对从高硅含铝物料中提取氧化铝具有重要意义。本文作者 研究高岭石与氧化铁在还原焙烧过程中的反应行为。热力学计算以及还原焙烧实验结果表明,由氧化铁还原得到的氧化亚铁优先与高岭石中的氧化铝反应而生成铝酸亚铁;与此同时,高岭石中的氧化硅转变为石英固溶体或者方石英固溶体。在有足量碳粉存在的条件下,过量的氧化亚铁和氧化硅反应生成的硅酸亚铁随着焙烧温度的升高进一步还原成氧化硅和金属铁。然而,升高焙烧温度及降低Fe2O3/Al2O3的摩尔比均能促进莫来石的形成。控制高岭土、赤铁矿和煤灰中Fe2O3/Al2O3/C摩尔比为1.2:2.0:1.2,在1373 K下还原焙烧60 min即可实现高岭石完全转变为独立的氧化硅和铝酸亚铁。研究结果有望为铝硅酸盐矿物中氧化铝和氧化硅的综合利用提供新技术。
关键词:高岭土;氧化铁;铝酸亚铁;石英固溶体;方石英固溶体;还原焙烧
(Edited by Wei-ping CHEN)
Foundation item: Project (51604309) supported by the National Natural Science Foundation of China
Corresponding author: Hong-yang WANG, Tel/Fax: +86-731-88830453, E-mail: hywang3@163.com;
Qiu-sheng ZHOU, Tel/Fax: +86-731-88830453, E-mail: qszhou@csu.edu.cn
DOI: 10.1016/S1003-6326(18)64927-1

