DOI: 10.11817/j.ysxb.1004.0609.2021-36596
湿法炼锌溶液中镁的水热结晶行为
熊甲成,李存兄,张利波,李世伟,夏 力,吴远桂,吉文斌,林晓坦
(昆明理工大学 冶金与能源工程学院,昆明 650093)
摘 要:利用高温下金属硫酸盐易结晶沉淀的化学原理,开展了水热结晶脱镁行为研究,研究了初始Zn2+浓度、反应温度、反应时间、初始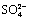 浓度、初始硫酸浓度对水热结晶脱镁过程的影响规律。结果表明:在初始Zn2+和Mg2+浓度分别为120 g/L和25 g/L、反应温度为200 ℃、反应时间3 h、初始
浓度、初始硫酸浓度对水热结晶脱镁过程的影响规律。结果表明:在初始Zn2+和Mg2+浓度分别为120 g/L和25 g/L、反应温度为200 ℃、反应时间3 h、初始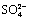 浓度为145 g/L、初始硫酸浓度为5×10-4 g/L的条件下,镁以MgSO4·6H2O的形式结晶析出,其脱出率可达到94.42%。升高反应温度以及初始
浓度为145 g/L、初始硫酸浓度为5×10-4 g/L的条件下,镁以MgSO4·6H2O的形式结晶析出,其脱出率可达到94.42%。升高反应温度以及初始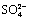 和Mg2+的浓度、延长结晶时间均有利于提高镁的结晶脱出率。受同离子效应影响,当Zn2+浓度由0增大至120 g/L可有效降低Mg2+的结晶析出温度,并使Mg2+的结晶脱出率增大40%以上,相近的ZnSO4·xH2O和MgSO4·xH2O微观结构抑制了水热条件下MgSO4·6H2O向MgSO4·H2O的转变。受初始硫酸浓度及溶液中
和Mg2+的浓度、延长结晶时间均有利于提高镁的结晶脱出率。受同离子效应影响,当Zn2+浓度由0增大至120 g/L可有效降低Mg2+的结晶析出温度,并使Mg2+的结晶脱出率增大40%以上,相近的ZnSO4·xH2O和MgSO4·xH2O微观结构抑制了水热条件下MgSO4·6H2O向MgSO4·H2O的转变。受初始硫酸浓度及溶液中 电离平衡引起
电离平衡引起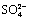 和H+浓度变化的影响,Mg2+的结晶析出率随初始硫酸浓度的升高先增大后减小。水热结晶除镁法可实现湿法炼锌含镁硫酸锌溶液中Mg2+的清洁高效脱出。
和H+浓度变化的影响,Mg2+的结晶析出率随初始硫酸浓度的升高先增大后减小。水热结晶除镁法可实现湿法炼锌含镁硫酸锌溶液中Mg2+的清洁高效脱出。
关键词:湿法炼锌;水热法;除镁;结晶;结晶脱出率
文章编号:1004-0609(2021)-06-1601-10 中图分类号:TF813 文献标志码:A
引文格式:熊甲成, 李存兄, 张利波, 等. 湿法炼锌溶液中镁的水热结晶行为[J]. 中国有色金属学报, 2021, 31(6): 1601-1610. DOI: 10.11817/j.ysxb.1004.0609.2021-36596
XIONG Jia-cheng, LI Cun-xiong, ZHANG Li-bo, et al. Hydrothermal crystallization of magnesium in zinc hydrometallurgical process[J]. The Chinese Journal of Nonferrous Metals, 2021, 31(6): 1601-1610. DOI: 10.11817/j.ysxb.1004.0609.2021-36596
我国85%以上的锌采用湿法炼锌工艺生产,在湿法炼锌过程中,硫化锌精矿中的镁溶解进入硫酸锌浸出液,并在湿法炼锌系统中循环富集[1-3]。湿法炼锌系统Mg2+含量增加将引起电解液黏度升 高,电导率下降,进而导致电流效率下降和电耗升高[4-6]。同时,Mg2+也会与Ca2+等其他杂质离子结晶析出,附着在运输管道内壁降低系统运输效率且不容易清理,镁的积累和有效排除一直是困扰湿法炼锌行业的难题。为了减小钙镁结晶给生产造成的危害,国内外大部分湿法炼锌企业采用中和沉淀除镁法和锌精矿酸洗除镁法进行生产系统镁的排除与控制。其中,中和沉淀除镁法是利用石灰中和部分废电解液,使其中的锌、镁一起沉淀,实现湿法炼锌系统绝大部分镁的开路,从而使体系Mg2+含量维持在15 g/L左右,但过程工艺冗长、成本较高[7];酸洗除镁法是部分高镁硫化锌精矿采用稀硫酸预浸脱镁,该方法能有效地除去硫化锌精矿中的镁,但硫化锌精矿中含有ZnO、ZnCO3时,这一部分的锌在酸洗时也进入到酸洗液中,造成回收困难[8]。除此之外,众多科研工作者开展了氟化物沉锌除镁法[9]、氨法沉锌排镁法[10-11]、高效萃取分离法[12]、碳酸盐沉淀法[13]的研究工作,如何消除引入的氟离子、氨气造成工作环境恶化、萃取剂有机物对湿法炼锌系统的危害仍是上述除镁方法需攻克的技术难题。
本研究根据金属硫酸盐在高温水热体系中结晶沉淀行为,以及硫酸镁、硫酸锌在高温下溶解度的差异开展高温水热结晶除镁的研究。以湿法炼锌企业产生的高镁硫酸锌浸出液成分特点为依据,研究了初始Zn2+浓度、反应温度、反应时间、初始 浓度、初始硫酸浓度等条件对水热结晶脱镁过程的影响规律,分析了MgSO4-ZnSO4-H2O体系硫酸镁竞争结晶机理,以期为湿法炼锌工业镁的净化脱出提供理论基础和技术指导。
浓度、初始硫酸浓度等条件对水热结晶脱镁过程的影响规律,分析了MgSO4-ZnSO4-H2O体系硫酸镁竞争结晶机理,以期为湿法炼锌工业镁的净化脱出提供理论基础和技术指导。
1 实验
1.1 实验试剂及料液
利用七水硫酸镁、七水硫酸锌、无水硫酸钠(分析纯,MgSO4·7H2O、ZnSO4·7H2O、Na2SO4,天津市恒兴化学试剂制造有限公司)、98%(质量分数,成都市科龙化工试剂厂)硫酸等化学试剂,根据湿法炼锌工业典型含镁硫酸锌溶液的成分特点配置不同成分的含镁硫酸锌初始料液。
1.2 实验方法
水热结晶除镁实验在配有加热罩、PID温度控制器、变速搅拌器和内部冷却盘管的2 L钛帕尔水热反应釜中进行。将1.2 L配置好的含镁硫酸锌溶液加入到水热反应釜内,密封后开始加热。当温度达到设定值时,开启搅拌并调节搅拌转速为300 r/min。按预设时间间隔,进行间断取样,并立即通过布赫纳真空过滤器过滤。实验结束后,用循环冷却水将水热反应釜冷却至40 ℃以下,开启水热釜并过滤反应料浆,结晶物80 ℃烘干和滤液一起进行分析检测。实验过程采用控制变量法,固定其他变量的情况下分别考察了反应温度、初始 浓度、初始H2SO4浓度、初始Zn2+浓度等因素对水热结晶脱镁过程的影响规律,通过改变PID温度控制器所设定温度改变反应温度;通过向溶液中加入Na2SO4调节反应初始
浓度、初始H2SO4浓度、初始Zn2+浓度等因素对水热结晶脱镁过程的影响规律,通过改变PID温度控制器所设定温度改变反应温度;通过向溶液中加入Na2SO4调节反应初始 浓度;通过向溶液中加入98%硫酸调节溶液初始硫酸浓度;通过向溶液中加入ZnSO4·7H2O调节溶液初始Zn2+浓度。
浓度;通过向溶液中加入98%硫酸调节溶液初始硫酸浓度;通过向溶液中加入ZnSO4·7H2O调节溶液初始Zn2+浓度。
1.3 分析检测与计算
采用原子吸收光谱仪(AAS)测定溶液中Mg2+的浓度。以二甲苯橙为指示剂,用EDTA滴定法测定溶液中Zn2+的浓度。采用特征X射线衍射(Cu Kα辐射,λ=0.1541838 nm,石墨单色器过滤器)检测分析结晶物的结晶形态及物相组成。利用扫描电子显微镜(SEM)分析主要元素(Mg、Zn、S、O)的分布,利用激光衍射粒度分析仪测定粒度分布。
采用公式(1)计算硫酸镁或硫酸锌的结晶脱出率:
 (1)
(1)
式中:η为硫酸镁或硫酸锌的结晶脱出率;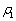 为初始溶液中Zn2+或Mg2+浓度,g/L;
为初始溶液中Zn2+或Mg2+浓度,g/L;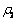 为实验过程取得样液中Zn2+或Mg2+浓度,g/L。
为实验过程取得样液中Zn2+或Mg2+浓度,g/L。
1.4 实验原理
1.4.1 水热结晶除镁基本原理
在含镁硫酸锌溶液水热结晶除镁过程中,溶液中的Mg2+和 结合结晶析出的过程分为MgSO4晶核形成及晶粒长大两个步骤。晶核形成的方式分为均相成核和异相成核[14]。
结合结晶析出的过程分为MgSO4晶核形成及晶粒长大两个步骤。晶核形成的方式分为均相成核和异相成核[14]。
当溶液中MgSO4的实际浓度 大于其溶解度S0,即MgSO4达过饱和状态时,它会以均相成核和异相成核的形式成核。具体可用图1所示的MgSO4-H2O系的S-T图[14]来表示。
大于其溶解度S0,即MgSO4达过饱和状态时,它会以均相成核和异相成核的形式成核。具体可用图1所示的MgSO4-H2O系的S-T图[14]来表示。

图1 MgSO4-H2O系的S-T图
Fig. 1 Solubility-temperature diagram of MgSO4-H2O
1) Ⅰ区为未饱和区,主要以Mg2+和 形式存在,体系处于式(2)所示动态平衡状态:
形式存在,体系处于式(2)所示动态平衡状态:
MgSO4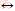 Mg2+(aq)+
Mg2+(aq)+ (aq) (2)
(aq) (2)
2) Ⅱ区为介安区,在此区域MgSO4浓度处于其溶解度周围,溶液接近过饱和状态,可发生异相成核,但不能自发形成能够稳定存在的晶核即均相成核,生成的MgSO4.nH2O结晶会返溶,此过程可用反应式(3)表示:
MgSO4·nH2O(s)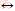 Mg2+(aq)+
Mg2+(aq)+ (aq)+nH2O (3)
(aq)+nH2O (3)
3) Ⅲ区为自动结晶区,在此区域MgSO4浓度大于其溶解度,溶液显著达过饱和状态,其过饱和度已足够自动形成核心,可按均相成核形式发生自动结晶过程,此过程可用反应式(4)表示:
Mg2+(aq) + (aq)+nH2O→MgSO4·nH2O(s) (4)
(aq)+nH2O→MgSO4·nH2O(s) (4)
故水热结晶除镁利用升高温度,硫酸镁溶解度显著下降,在过饱和状态下硫酸镁大量结晶析出的原理实现湿法炼锌系统镁的脱出与开路,从而消除因系统Mg2+浓度过高带来的不利影响。
1.4.2 金属硫酸盐溶解度曲线及物相组成演变
湿法炼锌工业含镁硫酸锌溶液的主要成分是含Zn2+(80~150 g/L),Mg2+(5~25 g/L)的金属硫酸盐。图2所示为MgSO4及ZnSO4在0~250 ℃温度范围内的溶解度曲线[15-16]。
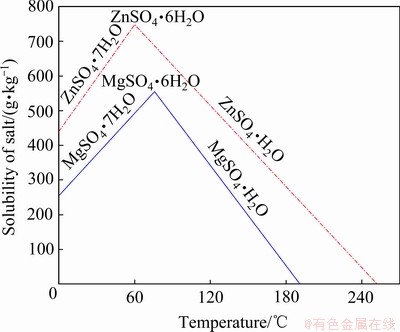
图2 0~250 ℃内MgSO4及ZnSO4的溶解度曲线
Fig. 2 Solubility curves of MgSO4 and ZnSO4 at temperature of 0-250 ℃
由图2可知,MgSO4和ZnSO4在相同温度下的溶解度有差异,在0~250 ℃的温度范围内MgSO4的溶解度均低于ZnSO4的溶解度,这为MgSO4的优先竞争析出创造了有利条件[17-18]。MgSO4和ZnSO4的溶解度随温度的变化趋势一致,溶解度随温度的升高先线性增大后线性减小。温度由0 ℃升高至250 ℃过程中,金属硫酸盐结晶产物随温度的变化发生脱水反应,依次生成:
MeSO4·7H2O→MeSO4·6H2O→MeSO4·H2O (Me: Mg或Zn) (5)
当反应体系温度超过80 ℃以后,随着温度的不断升高,MgSO4和ZnSO4的溶解度显著降低,在190 ℃和250 ℃下MgSO4和ZnSO4的溶解度几乎为零,可见,控制适当的反应温度可实现硫酸锌溶液中绝大部分镁的脱出,达到净化除镁的目的。
2 结果与讨论
2.1 反应温度的影响
在体系初始Mg2+浓度25 g/L、Zn2+浓度120 g/L、初始pH为5、搅拌转速300r/min、反应时间3h的条件下,研究了结晶温度(160~200 ℃)对水热硫酸镁结晶过程以及对脱镁结晶物相及成分、结晶率的影响规律,其结果如图3所示。由图3可知,溶液中Mg2+的浓度随着反应温度的升高而降低。在反应温度为180 ℃条件下,在整个反应过程中体系无明显结晶现象发生;当保温温度达到190 ℃时,反应时间为3 h时溶液中的Mg2+浓度由初始的25 g/L降低到14.72 g/L;当反应温度继续升高到200 ℃时,反应时间为1 h时,溶液中的Mg2+已经降低到了3.06 g/L,最终在反应时间为3 h时,溶液中的Mg2+降低到了1.39 g/L,可见在实验条件下反应体系温度超过190 ℃硫酸镁开始结晶析出,且随体系温度提高,结晶现象越来越明显,结晶率增大,升高高压除镁的反应温度有利于镁的结晶沉淀。
对不同温度条件下得到的除镁渣进行XRD分析,其结果如图4所示。由图4(a)可知,在不同温度下得到的结晶物都是由MgSO4·6H2O和ZnSO4·H2O两种物相组成。从图4(b)可以看出,随着反应温度的升高,MgSO4·6H2O主峰的强度逐渐增强,说明反应温度越高,越有利于硫酸镁结晶并发育长大,使MgSO4与ZnSO4的溶解度差异增大,从而使更多的镁结晶沉淀,而锌尽可能保留溶液中。
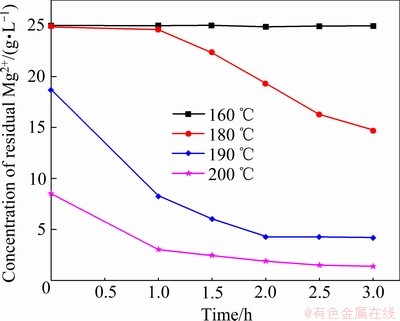
图3 反应温度对溶液中残留Mg2+浓度的影响
Fig. 3 Influence of reaction temperature on concentration of residual Mg2+ in solution
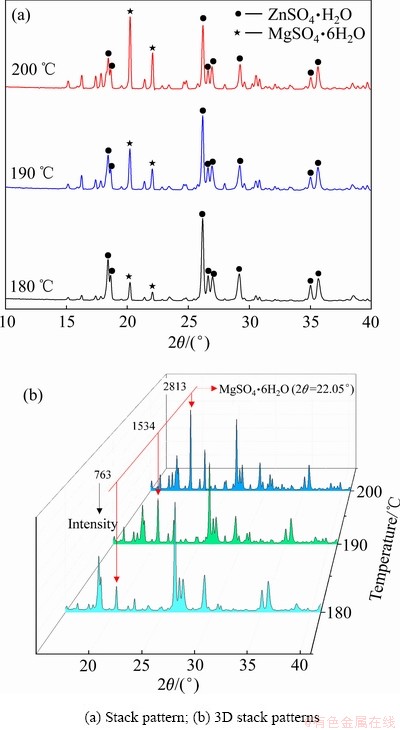
图4 不同反应温度下脱镁结晶物的XRD谱
Fig. 4 XRD patterns of precipitate at different reaction temperatures
不同反应温度下,除镁结晶物的SEM像及SEM-EDS分析如图5所示。由图5可知,200 ℃下除镁结晶物析出物主要由一些形状不规则的颗粒及团聚体组成,微域EDS分析发现,不同形貌结晶物均由硫酸镁和硫酸锌水合物组成。由图6可知,在180 ℃、190 ℃和200 ℃下均获得了形状不规则的颗粒状除镁结晶物,颗粒尺寸随反应温度的升高而减小。这是由于温度越高水热体系越易达过饱和状态,从而晶体成核速率大于其成长速率,导致其颗粒尺寸随温度升高而减小。
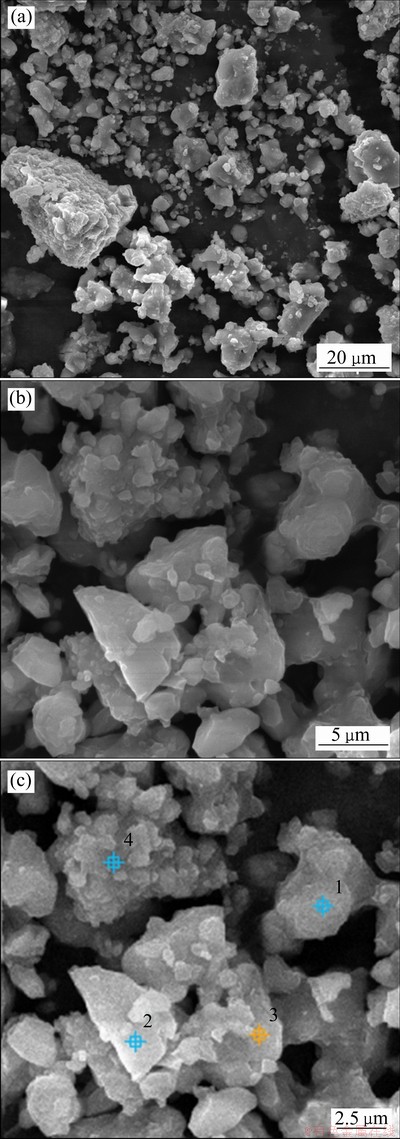
图5 200 ℃时脱镁结晶物的SEM像
Fig. 5 SEM images of precipitates produced at 200 ℃
表1 SEM-EDS点扫描元素质量分数
Table 1 Element mass fraction of SEM-EDS points-scan
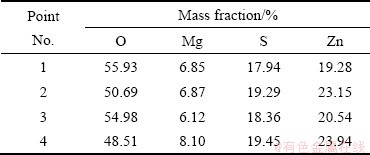

图6 不同反应温度下脱镁结晶物的SEM像
Fig. 6 SEM images of precipitates produced at different temperature
2.2 初始 浓度的影响
浓度的影响
水热体系中其他金属硫酸盐及其浓度变化对硫酸镁的结晶沉淀行为影响较为显著,在体系初始Mg2+浓度25 g/L、保温温度为180 ℃、初始硫酸浓度为5×10-4 g/L、搅拌转速300 r/min、反应时间3 h的条件下,研究了 浓度(25~145 g/L)对水热结晶除镁过程的影响规律。结果显示(见图7),溶液中残留Mg2+浓度随着初始
浓度(25~145 g/L)对水热结晶除镁过程的影响规律。结果显示(见图7),溶液中残留Mg2+浓度随着初始 浓度的升高而降低,当初始
浓度的升高而降低,当初始 浓度由25 g/L升高到145 g/L时,溶液中残留Mg2+浓度由25 g/L降低到14.72 g/L,镁的结晶脱出率不断增大。该过程可由以下反应式来解释:
浓度由25 g/L升高到145 g/L时,溶液中残留Mg2+浓度由25 g/L降低到14.72 g/L,镁的结晶脱出率不断增大。该过程可由以下反应式来解释:
Mg2++ +nH2O
+nH2O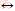 MgSO4·nH2O (6)
MgSO4·nH2O (6)

图7 初始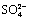 浓度对溶液中残留Mg2+浓度的影响
浓度对溶液中残留Mg2+浓度的影响
Fig. 7 Influence of initial 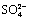 concentration on concentration of residual Mg2+ in solution
concentration on concentration of residual Mg2+ in solution
由溶度积规则[14]可知,溶液中的实际Mg2+浓度与 浓度的乘积为MgSO4的溶度积(Qc),当在固定温度下硫酸镁溶液达到平衡时,溶液中Mg2+浓度与
浓度的乘积为MgSO4的溶度积(Qc),当在固定温度下硫酸镁溶液达到平衡时,溶液中Mg2+浓度与 浓度的乘积为MgSO4的溶度积常数
浓度的乘积为MgSO4的溶度积常数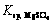 ,溶度积与溶度积常数的比值
,溶度积与溶度积常数的比值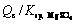 为硫酸镁在该温度下的过饱和率。不同初始
为硫酸镁在该温度下的过饱和率。不同初始 浓度下硫酸镁的Qc和
浓度下硫酸镁的Qc和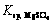 值如图8所示。由图8可知,随着水热体系
值如图8所示。由图8可知,随着水热体系 浓度的增加,MgSO4的溶度积(Qc)增大,
浓度的增加,MgSO4的溶度积(Qc)增大,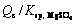 值增大,MgSO4结晶脱除率增大,溶液中残留Mg2+浓度降低。
值增大,MgSO4结晶脱除率增大,溶液中残留Mg2+浓度降低。

图8 不同初始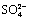 浓度下硫酸镁的Qc 和
浓度下硫酸镁的Qc 和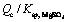
Fig. 8 Qc and 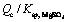 of MgSO4 at different initial
of MgSO4 at different initial 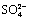 concentrations
concentrations
2.3 初始硫酸浓度的影响
在体系初始Mg2+浓度25 g/L、初始Zn2+ 120 g/L、反应温度为180 ℃、搅拌转速300 r/min、反应时间3 h的条件下,研究了溶液初始硫酸酸度(5×10-4~30 g/L)对水热结晶除镁过程的影响规律,初始硫酸浓度对溶液中残留Mg2+浓度变化的影响,其结果如图9所示。从图9可以看出,将初始硫酸酸度从5×10-4 g/L增加到5 g/L,溶液中残留Mg2+的浓度降低,而初始硫酸浓度从5 g/L增加到30 g/L时,溶液中残留Mg2+浓度略有增加。初始硫酸浓度对溶液中Mg2+浓度的影响可以由弱电解质在溶液中的电离平衡理论[19]来解释:
H2SO4=H++ (7)
(7)

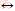 H++
H++ (8)
(8)
H2SO4作为强酸,在溶液中的电离如上述式(7)和式(8)所示,其第一步电离为完全电离,产生H+和 ;其第二步电离为
;其第二步电离为 的电离,为弱酸电离。
的电离,为弱酸电离。 的电离平衡常数以及解离度分别为弱酸电离平衡常数:
的电离平衡常数以及解离度分别为弱酸电离平衡常数:
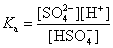 (9)
(9)
解离度:
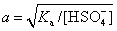 (10)
(10)
由HSC热力学数据库查得180 ℃时 的电离常数,并由式(10)计算
的电离常数,并由式(10)计算 的解离度a随
的解离度a随 浓度变化(5×10-4 g/L~30 g/L)的函数关系,其结果如图10所示。由图10可知,
浓度变化(5×10-4 g/L~30 g/L)的函数关系,其结果如图10所示。由图10可知, 的解离度a随
的解离度a随 浓度的升高而显著降低。因此,在初始硫酸浓度为5×10-4 g/L~5 g/L的低浓度范围内,
浓度的升高而显著降低。因此,在初始硫酸浓度为5×10-4 g/L~5 g/L的低浓度范围内, 的解离度较大,由
的解离度较大,由 电离出的
电离出的 增大了溶液中
增大了溶液中 的浓度,从而降低了硫酸镁的溶解度,使溶液中残留的Mg2+浓度降低。而当初始硫酸浓度继续增大时,溶液中的H+和
的浓度,从而降低了硫酸镁的溶解度,使溶液中残留的Mg2+浓度降低。而当初始硫酸浓度继续增大时,溶液中的H+和 浓度升高,
浓度升高, 的解离度a显著降低导致
的解离度a显著降低导致 电离产生的
电离产生的 减少,并且由式(8)平衡可知,当溶液中的H+浓度升高时溶液中的
减少,并且由式(8)平衡可知,当溶液中的H+浓度升高时溶液中的 浓度会进一步降低,从而导致硫酸镁的溶解度增大,溶液中残留的Mg2+浓度升高。
浓度会进一步降低,从而导致硫酸镁的溶解度增大,溶液中残留的Mg2+浓度升高。

图9 初始硫酸浓度对溶液中残留Mg2+浓度的影响
Fig. 9 Influence of initial sulfuric acid concentration on concentration of residual Mg2+ in solution
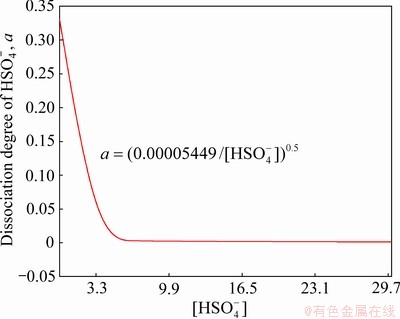
图10 180 ℃时 的解离度a与其浓度的关系图
的解离度a与其浓度的关系图
Fig. 10 Function relation of dissociation degree of  with [
with [ ] in 180 ℃
] in 180 ℃
2.4 初始Zn2+浓度的影响
在体系初始Mg2+浓度25 g/L、反应温度为180 ℃、溶液初始硫酸浓度为5×10-4 g/L、搅拌转速300 r/min、反应时间3 h的条件下,研究了初始Zn2+浓度(0~120 g/L)对高温水热硫酸镁结晶过程的影响规律,其结果如图11所示。由图11可知,反应温度为180 ℃、Zn2+浓度0 g/L时,镁的结晶脱出率接近0;当溶液中有Zn2+存在时,残留的Mg2+浓度随初始Zn2+浓度增加而降低。这是由于Zn2+与Mg2+价态相同,离子半径相似(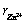 =74 pm、
=74 pm、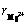 =72 pm),在溶液中产生同离子效应,导致MgSO4的溶解度随溶液中金属硫酸盐(MeSO4)浓度的增加而降低。从而,水热结晶脱镁体系中的Zn2+的存在可使硫酸镁结晶反应在较低温度下进行,并且Mg2+脱出率随体系Zn2+浓度的增加而增大。
=72 pm),在溶液中产生同离子效应,导致MgSO4的溶解度随溶液中金属硫酸盐(MeSO4)浓度的增加而降低。从而,水热结晶脱镁体系中的Zn2+的存在可使硫酸镁结晶反应在较低温度下进行,并且Mg2+脱出率随体系Zn2+浓度的增加而增大。
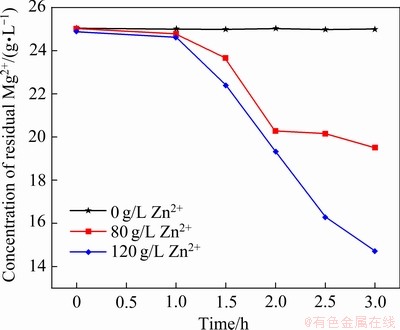
图11 初始Zn2+浓度对溶液中残留Mg2+浓度的影响
Fig. 11 Influence of initial Zn2+ concentration on concentration of residual Mg2+ in solution
采用X射线衍射对不同Zn2+浓度(0和120 g/L)的析出物进行了表征,结果如图12所示。由图12可知,在初始Zn2+浓度为0 g/L的条件下脱镁结晶物的物相主要包括MgSO4·6H2O和MgSO4·H2O,而在初始Zn2+浓度为120 g/L条件下脱镁结晶物的物相主要包括MgSO4·6H2O和ZnSO4·H2O。由MgSO4·6H2O 和ZnSO4·H2O的微观结构模拟结果(见图13)可知,MgSO4·6H2O与ZnSO4·H2O在结构上二者分别以各自的晶格为单位以一定的角度互相交错在一起,证明Zn2+的存在改变了MgSO4的析出物相形式。由于随着温度的升高,水合硫酸镁由MgSO4·6H2O转变为MgSO4·H2O,因此可以合理地推断,锌阻碍了MgSO4·6H2O向MgSO4·H2O的转化,使镁离子以MgSO4·6H2O的形式存在于析出物中。

图12 不同初始Zn2+浓度下脱镁结晶物XRD谱
Fig. 12 XRD patterns of precipitate at different Zn2+ concentrations

图13 190 ℃下脱镁结晶物的物相及MgSO4·6H2O-ZnSO4·H2O微观结构模型
Fig. 13 XRD patterns of precipitate in 190 ℃ and microscopic model of MgSO4·6H2O-ZnSO4·H2O
3 结论
1) 反应温度升高导致溶液中硫酸镁与硫酸锌溶解度差异增大,促进镁的结晶脱除。在反应体系温度超过190 ℃硫酸镁开始结晶析出,当反应温度为200 ℃、反应时间为3 h时,溶液中的残留Mg2+降低到了1.39 g/L;高温水热条件下,脱镁结晶物的颗粒尺寸随温度的升高而降低。
2) 增大初始 浓度,溶液中硫酸镁的过饱和率
浓度,溶液中硫酸镁的过饱和率 增大,促进镁离子结晶脱除。当溶液初始硫酸浓度低于5 g/L时,
增大,促进镁离子结晶脱除。当溶液初始硫酸浓度低于5 g/L时, 解离度较大,增大溶液初始硫酸浓度引起溶液中
解离度较大,增大溶液初始硫酸浓度引起溶液中 浓度增大,从而硫酸镁结晶脱除率增大;当溶液初始硫酸浓度大于5 g/L时,增大溶液中初始硫酸浓度会引起
浓度增大,从而硫酸镁结晶脱除率增大;当溶液初始硫酸浓度大于5 g/L时,增大溶液中初始硫酸浓度会引起 解离度降低,同时增大溶液中的H+浓度,受
解离度降低,同时增大溶液中的H+浓度,受 电离反应平衡的影响,溶液中的
电离反应平衡的影响,溶液中的 浓度降低,从而溶液中残留的Mg2+浓度增大,脱镁效率降低。
浓度降低,从而溶液中残留的Mg2+浓度增大,脱镁效率降低。
3) Zn2+会与溶液中Mg2+会产生同离子效应,增大初始Zn2+浓度导致溶液中金属硫酸盐(MeSO4)浓度的增加而使MgSO4的溶解度降低,促进镁的结晶脱出;Zn2+的加入阻碍了MgSO4·6H2O向MgSO4·H2O转化的过程,使镁离子以MgSO4·6H2O的形式存在于脱镁结晶渣中。
4) 在初始Zn2+和Mg2+浓度分别为120 g/L和25 g/L、反应温度为200 ℃、反应时间3 h、初始 浓度为145 g/L、初始硫酸浓度为5×10-4 g/L的条件下,镁以MgSO4·6H2O的形式结晶析出,其脱出率可达到94.42%。水热结晶除镁法可实现湿法炼锌含镁硫酸锌溶液中Mg2+的清洁高效脱出。
浓度为145 g/L、初始硫酸浓度为5×10-4 g/L的条件下,镁以MgSO4·6H2O的形式结晶析出,其脱出率可达到94.42%。水热结晶除镁法可实现湿法炼锌含镁硫酸锌溶液中Mg2+的清洁高效脱出。
REFERENCES
[1] 郑雪梅, 马爱元, 李 志, 等. 湿法炼锌中镁脱除现状与发展[J]. 世界有色金属, 2017(19): 6-7.
ZHENG Xue-mei, MA Ai-yuan, LI Zhi, et al. Current status and development of magnesium removal in zinc hydrometallurgy[J]. World nonferrous metals, 2017(19): 6-7.
[2] 马爱元, 郑雪梅. 湿法炼锌中微量杂质钙镁的来源及危 害[J]. 世界有色属, 2017(23): 5-6.
MA Ai-yuan, ZHENG Xue-mei. Sources and hazards of trace impurities calcium and magnesium in zinc hydrometallurgy[J]. World Nonferrous Metals, 2017(23): 5-6.
[3] 孙红燕, 森 维, 孔 馨. 钙镁结晶对湿法炼锌生产系统的影响研究[J]. 轻金属, 2016(1): 42-45.
SUN Hong-yan, SEN Wei, KONG Xin. Effect of calcium and magnesium crystallization on zinc hydrometallurgy production system[J]. Light metals, 2016(1): 42-45.
[4] 李永佳, 杨大锦, 曾桂生. 镁离子对锌电积液性质和电积能耗的影响[J]. 有色金属(冶炼部分), 2010(5): 2-4.
LI Yong-jia, YANG Da-jin, ZENG Gui-sheng. Effects of magnesium ion on properties of zinc electrodeposit and energy consumption in electrodeposition[J]. Non-ferrous Metals (Smelting Part), 2010(5): 2-4.
[5] GEORGALLI G A, EKSTEEN J J, PELSER M, et al. Fluoride based control of Ca and Mg concentrations in high ionic strength base metal sulphate solutions in hydrometallurgical circuits[J]. Minerals Engineering, 2008, 21(3): 200-212.
[6] BOOSTER J L, SANDWIJK A V, REUTER M A. Magnesium removal in the electrolytic zinc industry[J]. Minerals Engineering, 2000, 13(5): 517-526.
[7] 田 林, 谢 刚, 杨大锦. 镁对电积锌的影响研究[D]. 昆明: 昆明理工大学, 2010: 30-39.
TIAN Lin, XIE Gang, YANG Da-jin. Effect of Magnesium on zinc electrodeposition[D]. Kunming: Kunming University of Science and Technology, 2010: 30-39.
[8] 梅光贵, 王德润, 周敬元, 等. 湿法炼锌学[M]. 长沙: 中南大学出版社, 2001: 339.
MEI Guang-gui, WANG De-run, ZHOU Jing-yuan, et al. Zinc hydrometallurgy[M]. Changsha: Central South University Press, 2001: 339.
[9] BOOSTER J L, VAN SANDWIJK A, REUTER M A. Thermodynamic modelling of magnesium fluoride precipitation in concentrated zinc sulphate environment[J]. Minerals Engineering, 2001, 14(4): 411-422.
[10] СТОЛБОВА Е Ф, 张能成. 用氨法从锌生产工艺溶液中除去锰和镁的研究[J]. 湿法冶金, 1990(3): 71-73, 67.
СТОЛБОВА Е Ф, ZHANG Neng-cheng. Removal of Manganese and magnesium from zinc production process solution by ammonia method[J]. Hydrometallurgy, 1990(3): 71-73, 67.
[11] 郭天立, 王明辉, 未立清. 湿法炼锌工艺中钙镁等杂质脱除的方法探索[J]. 有色矿冶, 2007, 23(6): 34-36.
GUO Tian-li, WANG Min-hui, WEI Li-qing. Exploration on removal of calcium and magnesium impurities in zinc hydrometallurgy process[J]. Nonferrous Metallurgy, 2007, 23(6): 34-36.
[12] 李 春, 李自强, 刘小平. 溶剂萃取法从锌电积废液中分离钙镁的研究[J]. 有色金属(冶炼部分), 2000(6): 20-22.
LI Chun, LI Zi-qiang, LIU Xiao-ping. Study on separation of calcium and magnesium from zinc electrodeposited waste liquid by solvent extraction[J]. Non-ferrous Metals (Smelting Part), 2000(6): 20-22.
[13] LIN Qing-quan, GU Guo-hua, WANG Hui, et al. Separation of manganese from calcium and magnesium in sulfate solutions via carbonate precipitation[J]. Transaction of Nonferrous Metals Society of China, 2016, 26(4): 1118-1125.
[14] 马荣骏. 湿法冶金原理[M]. 北京: 冶金工业出版社, 2007: 206-209.
MA Rong-jun. Principle of hydrometallurgy[M]. Beijing: Metallurgical Industry Press, 2007: 206-209.
[15] ATHERTON S. Solubilities of inorganic and organic compounds: A compilation of solubility data from the periodical literature[M]. New York: Van Nostrand, 1953: 768.
[16] 刘慧杨, 邓志敢, 魏 昶, 等. 硫酸亚铁在高温水溶液中的结晶行为[J]. 中国有色金属学报, 2019, 29(11): 2645-2652.
LIU Hui-yang, DENG Zhi-gan, WEI Chang, et al. Crystallization of ferrous sulfate in high temperature aqueous solution[J]. The Chinese Journal of Nonferrous Metals, 2019, 29(11): 2645-2652.
[17] 张宇婕, 朱家文, 陈 葵, 等. 三元体系MgSO4-FeSO4- H2O的相平衡研究[J]. 化学工程. 2017, 45(1): 36-39.
ZHANG Yu-jie, ZHU Jia-wen, CHEN Kui, et al. Metastable phase equilibrium in the aqueous ternary system MgSO4- FeSO4-H2O[J]. Chemical Engineering, 2017, 45(1): 36-39.
[18] LI Jie, FAN Zhan-guo, LIU Yan-li, et al. Preparation of boric acid from low-grade ascharite and recovery of magnesium sulfate[J]. Transaction of Nonferrous Metals Society of China. 2010, 20(6): 1161-1165.
[19] 于定华, 张道化. 溶液与电离[M]. 合肥: 安徽教育出版社, 1987: 126-129.
YU Ding-hua, ZHANG Dao-hua. Solution and ionization[M]. Hefei: Anhui Education Press, 1987: 126-129.
Hydrothermal crystallization of magnesium in zinc hydrometallurgical process
XIONG Jia-cheng, LI Cun-xiong, ZHANG Li-bo, LI Shi-wei, XIA Li, WU Yuan-gui, JI Wen-bin, LIN Xiao-tan
(Faculty of Metallurgical and Energy Engineering, Kunming University of Science and Technology, Kunming 650093, China)
Abstract: The hydrothermal crystallization of magnesium sulfate was studied by using the chemical principle of easy crystallization precipitation of metal sulfate in high temperature. The effect of initial concentration of Zn2+, reaction temperature, reaction time, initial concentration of 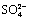 and initial concentration of sulfuric acid on hydrothermal crystallization process of de-magnesia was studied. The results show that, under the conditions of initial Zn2+ and Mg2+ concentrations of 120 g/L and 25 g/L, reaction temperature of 200 ℃, reaction time of 3h, and initial
and initial concentration of sulfuric acid on hydrothermal crystallization process of de-magnesia was studied. The results show that, under the conditions of initial Zn2+ and Mg2+ concentrations of 120 g/L and 25 g/L, reaction temperature of 200 ℃, reaction time of 3h, and initial 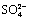 concentration of 145 g/L, magnesium is crystallized out in the form of MgSO4·6H2O, with release rate of 94.42%. Increasing the reaction temperature and the initial concentration of
concentration of 145 g/L, magnesium is crystallized out in the form of MgSO4·6H2O, with release rate of 94.42%. Increasing the reaction temperature and the initial concentration of 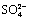 and Mg2+, and prolonging the crystallization time are beneficial to improving the crystallization rate of magnesium. Under the influence of the same ion effect, when the concentration of Zn2+ increases from 0 to 120 g/L, the crystallization temperature of Mg2+ can be effectively reduced and the crystallization rate of Mg2+ increases by more than 40%. The similar microstructures of ZnSO4·xH2O and MgSO4·xH2O prevent the transformation of MgSO4·6H2O into MgSO4·H2O under hydrothermal conditions. Under the influence of the initial sulfuric acid concentration and the concentration changes of
and Mg2+, and prolonging the crystallization time are beneficial to improving the crystallization rate of magnesium. Under the influence of the same ion effect, when the concentration of Zn2+ increases from 0 to 120 g/L, the crystallization temperature of Mg2+ can be effectively reduced and the crystallization rate of Mg2+ increases by more than 40%. The similar microstructures of ZnSO4·xH2O and MgSO4·xH2O prevent the transformation of MgSO4·6H2O into MgSO4·H2O under hydrothermal conditions. Under the influence of the initial sulfuric acid concentration and the concentration changes of 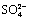 and H+ caused by the equilibrium of
and H+ caused by the equilibrium of  ionization in the solution, the crystallization rate of Mg2+ first increases and then decreases with the increase of the initial sulfuric acid concentration. The hydrothermal crystallization method of magnesium removal can realize the clean and efficient removal of Mg2+ from zinc sulphate solution containing magnesium.
ionization in the solution, the crystallization rate of Mg2+ first increases and then decreases with the increase of the initial sulfuric acid concentration. The hydrothermal crystallization method of magnesium removal can realize the clean and efficient removal of Mg2+ from zinc sulphate solution containing magnesium.
Key words: zinc hydrometallurgy; hydrothermal process; magnesium removal; crystallization; crystallization removal rate
Foundation item: Projects(52064034, 51664038, 51474117) supported by the National Natural Science Foundation of China; Project(2018BA078) supported by Key Research and Development of Yunnan Province, China
Received date: 2020-06-30; Accepted date: 2020-12-20
Corresponding author: LI Cun-xiong; Tel: +86-13518764748; E-mail: licunxiong@126.com
ZHANG Li-bo; Tel: +86-13888310177; E-mail: lbzhang@kmust.edu.cn
(编辑 李艳红)
基金项目:国家自然科学基金资助项目(52064034,51664038,51474117);云南省重点研发计划资助项目(2018BA078)
收稿日期:2020-06-30;修订日期:2020-12-20
通信作者:李存兄,教授,博士;电话:13518764748;E-mail:licunxiong@126.com
张利波,教授,博士;电话:13888310177;E-mail:lbzhang@kmust.edu.cn