AlCl3-BMIC离子液体的电导率
王喜然, 华一新, 赵秋凝, 李 艳
(昆明理工大学 材料与冶金工程学院, 昆明 650093)
摘 要: 研究AlCl3-BMIC(氯化-1-甲基-3-丁基咪唑)离子液体电导率与温度、 组分之间的关系。 结果表明: 当离子液体中AlCl3的摩尔分数x(AlCl3)〈0.667时, 离子液体的电导率随着x(AlCl3)的增加而增大; 但在0.667〈x(AlCl3)〈0.692区域内, x(AlCl3)对电导率影响不大; 随着温度的升高, AlCl3-BMIC离子液体的电导率增大, 电导率κ与温度t的关系符合Kohlraush经验公式; 在x(AlCl3)〈0.5的路易斯碱性区域, 温度系数α、 β均随着x(AlCl3)的增大而减小; 而在x(AlCl3)>0.5的路易斯酸性区域, x(AlCl3)对温度系数α、 β的影响均不明显。
关键词: AlCl3-BMIC离子液体; 氯化-1-甲基-3-丁基咪唑; 电导率 中图分类号:
文献标识码: A
Electrical conductivity of AlCl3-BMIC room temperature ionic liquids
WANG Xi-ran, HUA Yi-xin, ZHAO Qiu-ning, LI Yan
(Faculty of Materials and Metallurgical Engineering, Kunming University of Science and Technology, Kunming 650093, China)
Abstract: The electrical conductivities of AlCl3-BMIC (1-butyl-3-methylimidazolium chloride) ionic liquid were measured against temperature and composition. The results show that the conductivities of the ionic liquid increases with molar fraction x(AlCl3) of AlCl3 when x(AlCl3)〈0.667. However, the effect of AlCl3 molar fraction on the conductivities is negligible when 0.667〈x(AlCl3)〈0.692. It is demonstrated that the electrical conductivities are increased with increasing temperature, which can be described by Kohlraush empirical equation. For Lewis base ionic liquid (x(AlCl3)〈0.5), the temperature coefficients α and β are significantly decreased with increasing molar fraction of AlCl3. For Lewis acid ionic liquid (x(AlCl3)>0.5), however, the molar fraction of AlCl3has no obvious effect on the temperature coefficients.
Key words: AlCl3-BMIC ionic liquid; 1-butyl-3-methylimidazolium chloride; electrical conductivity
随着铝生产和消费的增长, 产生的废铝也在不断增加, 再生铝的精炼问题越来越受到人们的关注。 目前铝的精炼主要采用三层液高温熔盐电解法, 但此法存在电解温度高、 操作复杂、 能耗高、 设备腐蚀严重等缺点[1]。 离子液体的出现为铝的电解精炼提供了一种新的可能途径。
离子液体是室温离子液体的简称, 是由特定阳离子和阴离子构成的, 在室温或接近室温下呈液态的离子体系。 与其它溶剂相比, 室温离子液体具有宽的电化学窗口、 可室温操作、 热稳定性高、 导热导电性良好等优点[2-3]。 将其应用于金属的电沉积, 在室温下即可得到在高温熔盐中才能电沉积得到的金属或合金, 却没有高温熔盐那样的强腐蚀性, 且能耗大大降低; 同时, 在离子液体中进行电沉积, 副反应少, 因而得到的金属质量更好[4]。
目前, 研究者已经成功地从离子液体中电沉积出金属Al[5-9]及各种铝合金[10-14], 如Al-Cr、 Al-La、 Al-Ni、 Al-Mo、 Al-Mo-Mn等。 Kamavaram等[8]对AlCl3-BMIC离子液体做电解液进行再生铝的精炼做了研究, 电流密度可达200~500A/m2, 阴极电流效率为70%~90%, 铝的纯度大于98%。 这些工作表明, 将离子液体应用于电解精炼铝是可行的。 由于离子液体的电导率直接影响电解时的电流密度和电流效率, 而且研究电导率对于了解离子液体的性质及其结构有很大的价值, 所以本文作者对AlCl3-BMIC离子液体组成、 温度与电导率的关系进行研究。
1 实验
实验所用的氯代正丁烷、 无水三氯化铝、 N-甲基咪唑、 甲苯、 浓硫酸、 分子筛等为市售AR或CP试剂。 氯代正丁烷和N-甲基咪唑使用前分别加入相当其质量10%的分子筛除水, 然后进行蒸馏提纯; 甲苯先用分子筛除水, 再用浓硫酸萃取除去甲基噻吩, 然后蒸馏提纯。
合成氯化-1-甲基-3-丁基咪唑(BMIC)的反应方程式如式(1)所示:
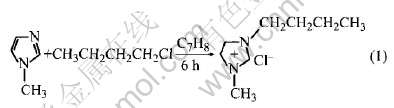
经纯化处理过的N-甲基咪唑和氯代正丁烷, 以摩尔比1∶1加入三颈瓶中, 甲苯作为溶剂, 在温度74℃, 氩气保护气氛下, 反应6~9h。 反应完毕后, 反应产物经分层除去大部分溶剂, 再经过减压蒸馏除去残余的溶剂和未反应物, 80℃下真空干燥3h, 得到淡黄色的粘稠性BMIC液体。
在充满氩气的手套箱中分别称取AlCl3和BMIC, 将AlCl3缓慢加入到BMIC中, 搅拌使其混合发生反应, 得到AlCl3-BMIC 离子液体。 该离子液体的合成反应为强放热反应, 反应速度很快。
在氩气保护气氛下及25~75℃的温度范围内, 采用DDS-11A型电导率仪测定AlCl3-BMIC离子液体的电导率。 为了准确测定离子液体的电导率, 实验时根据电导率的大小, 采用不同的电极。 当电导率在10-3~1S/m时, 使用DJS-1型铂黑电极; 当电导率大于1S/m时, 使用DJS-10型铂黑电极。
2 结果与分析
2.1 离子液体组成对电导率的影响
在恒温条件下, 实验测定了AlCl3-BMIC离子液体电导率与组成的关系, 其结果如图1所示。 为了便于比较, 现将Fannin等[15]的实验数据也示于图2。 由图可见, 在一定温度下, 离子液体的电导率与体系中AlCl3的摩尔分数x(AlCl3)有十分密切的关系, 其原因分析如下。
由于通过离子液体的电流是由离子的运动完成的, 所以电导率是所有带电体对电导率贡献的总和, 故电导率可以表示为[16]
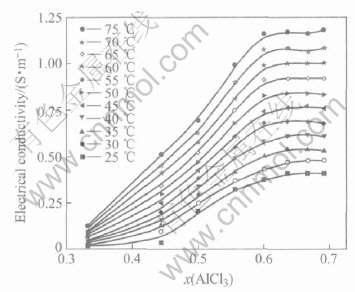
图1 本研究测定的离子液体的电导率与AlCl3摩尔分数的关系
Fig.1 Relationship between electrical conductivity and AlCl3 mole fraction by this work
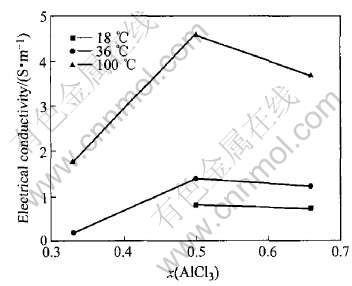
图2 Fannin等[15]测定的离子液体电导率与AlCl3摩尔分数的关系
Fig.2 Relationship between electrical conductivity and AlCl3 mole fraction by Fannin et al[15]

由式(2)和(3)可知, 电导率与带电体摩尔浓度及淌度呈正比, 而淌度与带电体半径及介质粘度成反比。 由于AlCl3的加入, 使得体系的组成、 带电体的数目、 半径及介质粘度都在不断变化, 从而引起电导率的变化。
在x(AlCl3)〈0.5的路易斯碱性区域内, 由本文测定的实验数据(图1)可知, 随着x(AlCl3)的增大, AlCl3-BMIC离子液体的电导率也随之增大, 这一结果与Fannin等[15]的实验数据(图2)一致。 由于AlCl3型离子液体的负离子存在形态主要取决于离子液体的组成, AlCl3-BMIC离子液体也不例外。 在x(AlCl3)〈0.5的碱性区, 离子液体中的负离子Cl-与加入的AlCl3反应[2]:
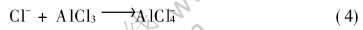
反应生成 AlCl-4负离子。 因此, 在碱性区, 离子液体中存在负离子Cl-和AlCl-4。 如果每个正离子与两个负离子配位, 则此区域内可能存在的低聚体有:
…Cl--BMI+-Cl-… ;
…Cl--BMI+-AlCl-4… ;
...AlCl-4-BMI+-AlCl-4…
这些表达式仅代表低聚体的一部分[17]。 低聚体实际是由正负离子交替排列形成的链状聚合体, 链的长度与正负离子的种类、 离子间的相互作用力及氢键的强弱有关。 虽然正负离子之间的作用力比较弱, 但足以使原来单个的离子失去其独立性, 从而使低聚体或带正电、 或带负电、 或呈电中性。 负离子体积越小, 聚合度越高, 链越长, 低聚体体积相对就大; 反之, 正负离子体积越大, 聚合度就越低, 链越短, 低聚体体积相对要小。 未加AlCl3前, 由于BMIC中Cl-的体积小, 氢键作用和离子缔合效应大, 有利于形成低聚体…Cl-BMI+-Cl-…, 且低聚体的聚合度比较高, 带电体数目少。 随着AlCl3的加入, 氢键的网状结构被破坏, 氢键的受体Cl-被AlCl-4 取代, 氢键作用减弱, 负离子Cl- 减少, 低聚体…Cl--BMI+-Cl-…减少; 同时, AlCl-4增多, 低聚体…Cl--BMI+-AlCl-4…及…AlCl-4-BMI+-AlCl-4…增多, 且聚合度降低, 实际带电体的数目增多, 带电体半径r变小, 离子液体粘度η降低, 带电体淌度ui增大, 离子液体电导率也随之增大。
在0.5〈x(AlCl3) 〈0.667区域内, 由本文的数据(图1)可知, AlCl3-BMIC离子液体的电导率仍然随x(AlCl3)的增加而增大。 在此区域内, 离子液体呈路易斯酸性, 体系中的负离子AlCl-4与加入的AlCl3 反应[2] :
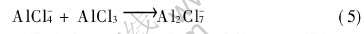
反应生成Al2Cl-7负离子。 随着AlCl3的加入, 负离子AlCl-4减少, 大量的负离子Al2Cl-7生成, 氢键作用减弱直至消失, 离子缔合效应继续减弱, 低聚体…AlCl-4-BMI+-AlCl-4…减少, …AlCl-4-BMI+-Al2Cl-7…及…Al2Cl-7-BMI+-Al2Cl-7…增多。 虽然Al2Cl-7的体积比AlCl-4的体积大, 但聚合度低, 实际运动带电体的体积变小, 故随着x(AlCl3)的增大, 离子液体的粘度η降低, 带电体数目增多, 离子淌度ui变大, 电导率随之增大。 这种分析与实验结果是一致的。 但从Fannin等[15]的实验数据看, 当x(AlCl3)>0.5时, AlCl3-BMIC离子液体的电导率随x(AlCl3)的增加呈下降趋势, 其结果与本实验数据不吻合。 本实验在x(AlCl3)>0.5的区域测定了5个点, 而Fannin等只测定了1个点, 从统计角度来看, 本实验的结果应该更可靠一些。
在x(AlCl3) >0.667区域内, 由本实验数据(图1)可知, 体系中AlCl3的摩尔分数对AlCl3-BMIC离子液体的电导率已经没有明显的影响。 在此区域内, 离子液体中的阴离子主要为Al2Cl-7, 进一步在离子液体中增加AlCl3, 可能会产生少量的Al3Cl-10 [2]:
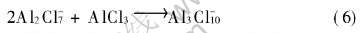
液体中另有少量Al2Cl6分子存在。 但由于Al3Cl-10阴离子和Al2Cl6分子的量非常少, 对电导率的影响很小, 故体系的电导率主要决定于Al2Cl-7阴离子的数量。 所以在0.667〈x(AlCl3) 〈0.692的区域, AlCl3的摩尔分数对电导率的影响很小。
2.2 温度对电导率的影响
本实验数据表明, 在离子液体组成一定的条件下, AlCl3-BMIC离子液体的电导率随温度的升高而增大, Fannin等[15]的实验数据也表明了这一点。 这是因为离子液体由阴、 阳离子组成, 依靠离子迁移来导电, 随着温度的升高, 离子能量的增加, 使得离子容易克服相互间的聚合效应和氢键作用, 导致离子液体粘度降低, 离子运动的阻力减小, 在电场作用下迁移速度加快, 电导率增大。
对图1中的实验数据进行回归分析发现, 离子液体电导率与温度的关系符合Kohlraush经验式:

在一定的AlCl3摩尔分数条件下, AlCl3-BMIC离子液体的电导率与温度的关系如图3所示。 由图3可以计算出Kohlraush经验式(7)中的κ0、 α和β, 其结果列于表1。
表1 AlCl3-BMIC离子液体电导率与温度回归方程(式(7))的相关参数
Table 1 Parameters for Eqn.(7) obtained by regression
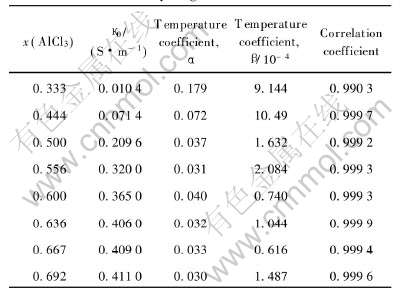
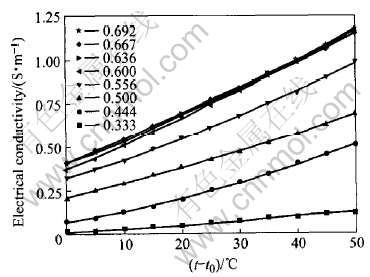
图3 不同x(AlCl3)下离子液体电导率与温度的关系
Fig.3 Relationship between electrical conductivity and temperature at various x(AlCl3)
分别用温度系数α、 β对AlCl3的摩尔分数作图, 可以得到温度系数与x(AlCl3)的关系, 如图4和图5所示。 从图4可以看出, 在x(AlCl3)〈0.5的路易斯碱性区, 随着x(AlCl3)的增大, 温度系数α明显下降; 在x(AlCl3) >0.5的路易斯酸性区, α的下降趋势趋于平缓, 说明x(AlCl3)对α的影响越来越小。 α的这种变化规律与AlCl3-BMIC离子液体中的氢键作用和缔合效应有关。 体系的氢键作用和缔合效应越大, 温度对离子液体的结构影响越大。 由于增加AlCl3-BMIC离子液体中的AlCl3摩尔分数会减少体系中的氢键作用和缔合效应, 因而增加x(AlCl3)会降低温度对氢键作用和缔合效应的影响, 并使体系的温度系数α变小。 在x(AlCl3)〈0.5的区域, 由于体系的氢键作用和缔合效应较大, 故x(AlCl3)对温度系数α的影响比较大; 而在x(AlCl3)>0.5的区域, 由于体系的氢键作用和缔合效应较小, 故x(AlCl3)对温度系数α的影响比较小。
从图5可以看出, 在x(AlCl3)〈0.5的区域,
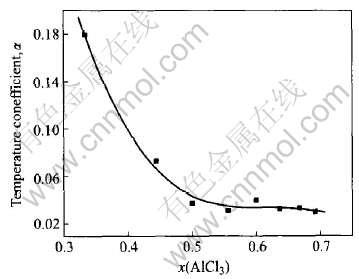
图4 温度系数α与x(AlCl3)的关系
Fig.4 Effect of x(AlCl3) on α
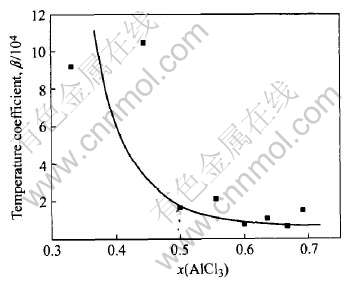
图5 温度系数β与AlCl3摩尔分数的关系
Fig.5 Effect of x(AlCl3) on β
温度系数β随着x(AlCl3)的增大而下降; 在x(AlCl3)>0.5的区域, x(AlCl3)对于β的影响不明显。 温度系数β随x(AlCl3)变化的原因与温度系数α的情形类似。
3 结论
1) 在x(AlCl3)〈0.667的区域内, 随着x(AlCl3)的增加, 离子液体电导率升高; 在0.667〈x(AlCl3)〈0.692的区域, x(AlCl3)对电导率的影响很小。
2) 温度升高, 离子液体的电导率随之升高, 电导率与温度的关系符合Kohlraush经验式。
3) 在 x(AlCl3)〈0.5的区域内, 离子液体中x(AlCl3)对温度系数α、 β影响较大; 在 x(AlCl3)>0.5的区域, x(AlCl3)对α、 β影响比较小。
REFERENCES
[1]杨重愚. 轻金属冶金学[M]. 北京: 冶金工业出版社, 1991: 200-204.
YANG Zhong-yu. Metallurgy of Light Metals[M]. Beijing: Metallurgical Industry Press, 1991: 200-204.
[2]李汝雄. 绿色溶剂-离子液体的合成及应用[M]. 北京: 化学工业出版社, 2004: 10-27.
LI Ru-xiong. Green Solvent-Synthesis and Application of Ionic Liquid [M]. Beijing: Chemical Industry Press, 2004: 20-27.
[3]Welton T. Room-temperature ionic liquids. Solvents for synthesis and catalysis[J]. Chem Rev, 1999, 99: 2071-2083.
[4]Endres F. Ionic liquids: solvents for the electrodeposition of metals and semiconductors[J]. Chem Physchem, 2002 , 3: 144-154.
[5]ZHANG Ming-ming, Kamavaram V, Reddy R G. New electrolytes for aluminum production: Ionic liquids[J]. JOM, 2003, 55(11): 54-57.
[6]ZHAO Yu-guang, Van der Noot T J. Electrodeposition of aluminum from room temperature AlCl3-TMPAC molten salts[J]. Electrochimica Acta, 1997, 42(11): 1639-1643.
[7]LIAO Qing, Pitner W R, Stewart G, et al. Electrodeposition of aluminum from the aluminum chloride-1-methy-3-ethylimidazolium chloride room temperature molten salt+benzene[J]. J Electrochem Soc, 1997, 144(3): 936-943.
[8]Kamavaram V, Mantha D, Reddy R G. Recycling of aluminum metal matrix composite using ionic liquids: effect of process variables on current efficiency and deposit characteristics[J]. Electrochmica Acta, 2005, 50: 3286-3295.
[9]逢清强. AlCl3-BPC中电镀铝的研究[D]. 杭州: 浙江大学, 2000.
FENG Qing-qiang. Electroplating of Aluminum from AlCl3-BPC Melt [D]. Hangzhou: Zhejiang University, 2000.
[10]Ali M R, Nishikata A, Tsuru T. Electrodeposition of aluminum-chromium alloys from AlCl3-BPC melt and its corrosion and high temperature oxidation behaviors[J]. Electrochimica Acta, 1997, 42(15): 2347-2354.
[11]Tsuda T, Nohira T, Ito Y. Nucleation and surface morphology of aluminum lanthanum alloy electrodeposited in a LaCl3-saturated AlCl3-EtMeImCl room temperature molten salt[J]. Electrochimica Acta, 2002, 47(17): 2817-2822.
[12]Ali M R, Nishikata A, Tsure T. Electrodeposition of Al-Ni intermetallic compounds from aluminum chloride-N-(n-butyl) pyridinium chloride room temperature molten salt[J]. Journal of Electroanalytical Chemistry , 2001, 513: 111-118.
[13]Tsuda T, Hussey C L, Staffoud G R. Electrodeposition of Al-Mo alloys from the lewis acidic aluminum chloride-2-ethyl-3-methylimidazolium chloride molten salt[J]. J Electrochem Soc, 2004, 152(9): C379-C384.
[14]Tsuda T, Hussey C L, Staffoud G R. Electrodeposition of Al-Mo-Mn ternary alloys from the lewis acidic AlCl3-EtMeImCI molten salt[J]. J Electrochem Soc, 2005, 152(9): C620-C625.
[15]Fannin A A Jr, Floreani D A, King L A, et al. Properties of 1, 3-dialkylimidazolium chloride-aluminum chloride ionic liquids. 2. phase transitions, densities electrical conductivities and viscosities[J]. J Phys Chem, 1984, 88: 2614-2624.
[16]高颖, 邬冰. 电化学基础[M]. 北京: 化学工业出版社, 2004: 11-14.
GAO Ying, WU Bing. Foundations of Electrochemistry[M]. Beijing: Chemical Industry Press, 2004: 11-14.
[17]Wikes J S, Frye J S, Reynolds G. 27Al and 13C NMR Studies of aluminum chloride-dialkylimidazolium chloride molten salts[J]. Inorg Chem, 1983, 22(26): 3870-3872.
(编辑何学锋)
基金项目: 国家自然科学基金资助项目(50564006); 云南省自然科学基金重点项目(2005E0004Z)
收稿日期: 2006-05-12; 修订日期: 2006-10-10
通讯作者: 华一新, 博士, 教授; 电话: 0871-5162008; E-mail: huayixin@public.km.yn.cn