铝基体上碳纳米管原位均匀合成及其复合材料的性能
来源期刊:中国有色金属学报(英文版)2014年第7期
论文作者:李海鹏 范佳薇 康建立 赵乃勤 王雪霞 李宝娥
文章页码:2331 - 2336
关键词:铝基复合材料;碳纳米管;化学气相沉积;原位合成
Key words:aluminum matrix composites; carbon nanotubes; chemical vapor deposition; in-situ synthesis
摘 要:采用负载于铝粉上的镍催化剂,成功地在650 °C通过化学气相沉积法在铝基体中原位合成碳纳米管。结构表征表明,所合成的碳纳米管具有较高的石墨化程度和平直的石墨壳层。通过该方法实现铝粉中碳纳米管的弥散分布,其分散效果优于传统机械混合方法。利用所合成的碳纳米管/铝原位复合粉末,采用粉末冶金工艺制备碳纳米管/铝基复合材料。性能测试表明,制备的复合材料的力学性能和尺寸稳定性得到显著提高,其原因在于铝基体中碳纳米管的均匀分散和碳纳米管-铝基体之间良好的界面结合。
Abstract: Using nickel catalyst supported on aluminum powders, carbon nanotubes (CNTs) were successfully synthesized in aluminum powders by in-situ chemical vapor deposition at 650 °C. Structural characterization revealed that the as-grown CNTs possessed higher graphitization degree and straight graphite shell. By this approach, more homogeneous dispersion of CNTs in aluminum powders was achieved compared with the traditional mechanical mixture methods. Using the in-situ synthesized CNTs/Al composite powders and powder metallurgy process, CNTs/Al bulk composites were prepared. Performance testing showed that the mechanical properties and dimensional stability of the composites were improved obviously, which was attributed to the superior dispersion of CNTs in aluminum matrix and the strong interfacial bonding between CNTs and matrix.
Trans. Nonferrous Met. Soc. China 24(2014) 2331-2336
Hai-peng LI1, 2, Jia-wei FAN2, Jian-li KANG3, Nai-qin ZHAO1, Xue-xia WANG2, Bao-e LI2
1. School of Materials Science and Engineering, Tianjin University, Tianjin 300072, China;
2. School of Materials Science and Engineering, Hebei University of Technology, Tianjin 300130, China;
3. School of Materials Science and Engineering, Tianjin Polytechnic University, Tianjin 300387, China
Received 17 October 2013; accepted 11 April 2014
Abstract: Using nickel catalyst supported on aluminum powders, carbon nanotubes (CNTs) were successfully synthesized in aluminum powders by in-situ chemical vapor deposition at 650 °C. Structural characterization revealed that the as-grown CNTs possessed higher graphitization degree and straight graphite shell. By this approach, more homogeneous dispersion of CNTs in aluminum powders was achieved compared with the traditional mechanical mixture methods. Using the in-situ synthesized CNTs/Al composite powders and powder metallurgy process, CNTs/Al bulk composites were prepared. Performance testing showed that the mechanical properties and dimensional stability of the composites were improved obviously, which was attributed to the superior dispersion of CNTs in aluminum matrix and the strong interfacial bonding between CNTs and matrix.
Key words: aluminum matrix composites; carbon nanotubes; chemical vapor deposition; in-situ synthesis
1 Introduction
Since the discovery in 1991 by IIJIMA [1], carbon nanotubes (CNTs) with unique structure have been the focus of numerous investigations because of their fascinating properties and application potentials. They are regarded as excellent reinforcements for composites to overcome the performance limits of conventional materials [2]. Many research efforts have dealt with CNTs/polymer, CNTs/ceramic and CNTs/metal composites [3-5]. Most researches in this field have dealt with CNT/polymer composites, which show obvious property enhancement [6,7]. However, compared with polymer matrix composite, the agglomeration of CNTs in metal or ceramic composites is more severe and the interfacial wettability between matrix and reinforcement is far from satisfaction, due to the strong van der Waal’s force among CNTs caused by their small scale and large specific surface area. Thus, the reinforcement effect of CNTs is not fully exploited. In order to remedy those problems, some researchers tried the methods, such as ball milling, plasma spraying, to achieve the homogenous dispersion of CNTs in the composites. However, the perfect structure of CNTs may be destroyed, and the dispersion of CNTs as well as the properties of the composites is still not ideal.
With the development of in-situ synthesis technique in CNTs/ceramic composites [8,9], some researchers paid particular attention to its application in CNTs/metal composites. CNTs have been successfully synthesized in Cu, Mg, Ag and Al matrixes by in-situ chemical vapor deposition (CVD) [10-13], which demonstrated the feasibility of in-situ synthesis of CNTs in metal matrixes. However, the low melting point and active chemical property of the metals, such as Al and Mg, restrict the reaction temperature of CNTs growth by CVD in these metals, resulting in the poor graphitization degree and aggregation of CNTs. Accordingly, the properties of CNTs/metal composites are still not satisfactory. And the in-situ synthesis of CNTs in low-melting-point metals is still under discussion.
In our previous work, the feasibility of in-situ synthesis of CNTs in aluminum powders was confirmed [10] and aluminum matrix composite reinforced by CNTs with diverse structures was fabricated by in-situ synthesis process [14]. However, it is still unclear that which type of transitional metal catalyst is more suitable to prepare CNTs/Al matrix composites with superior performance. In this work, the synthesis effects of CNTs, using different transition metal (Fe, Co and Ni) catalysts supported on aluminum powders at the critical temperature approaching the melting point of aluminum, were first investigated. The transitional metal catalyst suitable to in-situ synthesis of CNTs in low-melting- point metal was determined. The yield, graphitization degree and dispersion of CNTs in aluminum powders were analyzed. CNTs/Al in-situ composites were prepared and characterized. By this work, the in-situ synthesis process of CNTs/Al composite powders was optimized and the performance improvement of aluminum matrix composite was realized.
2 Experimental
2.1 Preparation of CNTs/Al in-situ composite powders
Right amount of pure Al powder was dissolved into Fe(NO3)3·9H2O, Co(NO3)2·6H2O and Ni(NO3)2·6H2O aqueous solutions with a concentration of 0.1 mol/L, respectively. Then 0.1 mol/L NaOH aqueous solution was dripped gradually into the above mixtures with constant stirring for 0.5 h. The co-precipitation was aged at room temperature for 12 h, filtrated and dried. Binary colloid Me(OH)x/Al (Me represents Fe3+, Co2+ or Ni2+) was obtained. Then, the Me(OH)x/Al powders were calcined in N2 atmosphere at 450 °C for 2 h and the transition metal oxide/Al catalyst precursors were obtained. The catalyst precursors were put in quartz crucible and then placed into horizontal quartz tube reactor. The precursors were heated to 650 °C in N2 atmosphere, and then reduced in a H2 atmosphere for 2 h. Then CNTs growth was performed in a mixture gas of CH4+N2 (V(CH4)=60 mL/min, V(N2)=420 mL/min) at 650 °C for 60 min. Finally, the reactor was cooled to room temperature in N2 atmosphere. The yield of the carbon nanostructure is defined as
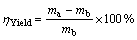 (1)
(1)
where ηYield is the yield of in-situ synthesized carbon nanostructure, ma is the mass of as-grown composite powder, and mb is the mass of catalyst precursor.
2.2 Preparation of CNTs/Al bulk composites
The CNTs/Al bulk composites were prepared by powder metallurgy process. The CNTs/Al in-situ composite powders were pressed in a mold of diameter 20 mm under a pressure of 450 MPa. Subsequently, the obtained bulk composites were sintered at 610 °C for 2 h in a vacuum sintering furnace. Then they were re-pressed by a hot-extrusion mold with an extrusion ratio of 10:1 under a pressure of 600 MPa at 450 °C. Pure Al bulk was prepared under the same processing conditions for comparison.
2.3 Characterization
Surface morphology and microstructure of the as-grown carbon nanostructures were examined by field emission scanning electron microscope (FESEM, Hitachi S-4800) and high-resolution transmission electron microscope (HRTEM, Philips TECNAI G2 F20, 200 kV). X-ray diffractometer (XRD, Rigaku D/max 2000V/pc) was employed to detect the phase composition of the as-grown composite powders. The hardness of CNTs/Al composites was measured using a hardness testing device (Everone MH-6) operated at the load of 0.49 N and dwell time of 30 s. Tensile test of the round specimens of 2.5 mm in diameter was performed by a Shimadzu Universal Testing Machine model AG-50kNG (M/s Shimadzu, Kyoto, Japan). The thermal expansion of the composites was determined using NETZSCH DIL 402C thermo- mechanical analyzer at a heating rate of 5 °C/min within the temperature range from 50 to 300 °C.
3 Results and discussion
3.1 Characterization of CNTs/Al in-situ composite powders
The morphology and structure of the as-grown composite powders, synthesized by Fe, Co and Ni/Al catalysts with 10% transition metal content, were characterized by SEM and TEM, as shown in Fig. 1. Though the yield of the composite powders varied with various transition metal contents (5% and 15%), the type, morphology and structure of the as-grown carbon nanostructures were similar. Figure 1(a) shows the SEM image of the CNTs/Al composite powders prepared by Fe/Al catalyst. A mass of entangled CNTs could be observed and their diameters were not uniform, ranging from 10 to 30 nm. Their average length was about 1 μm. The surfaces of CNTs seemed not clean and smooth. When the reaction time was 60 min, the yield of CNTs was 2.7%. Due to the low yield, no obvious diffraction peaks of graphite appeared in the XRD pattern inserted in Fig. 1(a). TEM analysis revealed the microstructure of these CNTs, as shown in Fig. 1(d). The CNTs had relatively large hollowness and their wall thickness was 5-8 nm. Measured by Digital Micrograph software, the average interplanar spacing was 0.346 nm, which was far from the ideal (002) interplanar spacing (0.34 nm) of graphite carbon, which suggested that the CNTs possessed more defects and less crystallinity. Meanwhile, many imperfect graphitic layers existed at the exterior of CNTs. It was well known that aluminum carbide (Al4C3) was a common compound formed during the fabrication of Al/C (graphite and carbon fibers) composites because of relatively low free energy of formation (-53.09 kJ at 298 K) [15]. CI et al [16] investigated the interfacial reaction between CVD CNTs and Al, and found that Al4C3 preferred to form and grow along the graphitic prism planes or defects. And our recent research [14] also suggested that the CNTs, with more imperfect graphitic layers or graphitic prism planes, were easy to react with Al matrix, which deteriorated the properties of CNTs/Al composites. Thus, Fe might not be the most suitable transition metal to synthesize high-quality CNTs in Al matrix. Figures 1(b) and (e) show the SEM and TEM images of the product fabricated by Co/Al catalyst, respectively. From Fig. 1(b), it could be found that the as-grown carbon nanostructures were mainly spherical nanoparticles, ranging in diameter from 30 to 100 nm. The diffraction peaks of graphite were not observed in the XRD pattern (insert in Fig. 1(b)) because of the lower yield (1.59%). Figure 1(e) further confirmed that the products were carbon-coated cobalt nanoparticles, rather than CNTs, inferring that Co catalyst could not synthesize CNTs successfully under the experimental conditions. This might be due to the lower synthesis temperature and the active chemical property of Al matrix. Figures 1(c) and (f) show the SEM and TEM images of the carbon nanostructures synthesized by Ni/Al catalyst. As shown in Fig. 1(c), lots of CNTs, with clean, smooth tube wall and high purity, were successfully synthesized. Especially, the as-grown CNTs had relatively straight tube wall, which was easy to realize the homogeneous dispersion of CNTs in Al matrix. When the synthesis time was 60 min, the yield of CNTs was 26.8%. In the XRD pattern inserted in Fig. 1(c), except the peaks belonging to Al and Ni, obvious diffraction peak of graphite (002) could be observed at 2θ=26.2°, which was close to the characteristic peak of graphite (002) at 2θ=26.4°. The TEM image in Fig. 1(f) shows the graphitic sheets of CNTs were apparent and perfect. The interlayer spacing of 0.341 nm was similar to the ideal (002) interplanar spacing (0.34 nm) of graphite carbon, which demonstrated that the CNTs were well-graphitized.
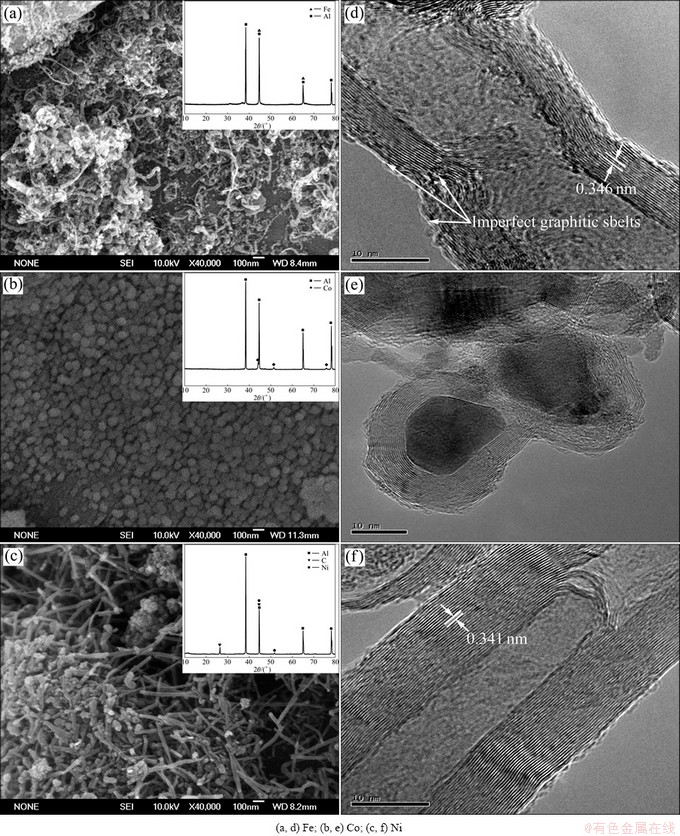
Fig. 1 SEM (a, b, c) and TEM (d, e, f) images of carbon nanostructures synthesized by different transition metal/Al catalysts with content of 10%
To acquire CNTs with ideal structure, homogeneous dispersion and high yield in Al matrix, choosing optimal catalyst type is very important. Compared with Fe/Al and Co/Al catalysts, Ni/Al catalyst could realize in-situ homogeneous synthesis of CNTs, as well as ideal structure and high yield. Herein, the difference of synthesizing CNTs with different transition metals could be attributed to the lower CVD temperature, active property of Al matrix and catalytic activity of transition metal. Restricted by the low melting point of Al matrix, the synthesis temperature of CNTs in Al powders must be below 660 °C, much lower than the synthesis temperature when using ceramic carrier, such as Al2O3, SiO2. Meanwhile, intermetallic compounds might be formed between Fe, Co, Ni and Al according to the phase diagram. They cause the difficulty for these transition metals to fully display catalytic roles. Under the circumstances, Ni has the strongest catalytic activity and the highest CNT growth rate among the three transition metals [17,18]. Thus, it is reasonable for Ni/Al catalyst to synthesize CNTs with higher degree of graphitization, perfect structure and higher yield.
3.2 Thermal expansion of CNTs/Al composites
The original results of thermal expansion test of CNTs/Al composites were obtained in the form of a thousandth linear change (TLC) versus temperature curve. Figure 2 shows the TLC versus temperature curves of pure Al bulk and CNTs/Al in-situ composites, revealing a noticeable improvement in the dimensional stability of Al matrix. For example, the TLC of Al matrix decreased by 0.34×10-3 at 300 °C when the content of CNTs in the composite was 0.25%. With increasing the content of CNTs to 0.75%, the TLC decreased by 0.99×10-3, indicating the enhanced dimensional stability of Al matrix. Figure 3 shows the variation of coefficient of thermal expansion (CTE) versus temperature, which was determined at an interval of 50 °C based on the calculated slope fit between two selected temperatures on the TLC versus temperature curve. With the rise of temperature, the CTEs of pure Al and CNTs/Al composites increased rapidly in the temperature range of 100-200 °C, due to the change of the elastic modulus of Al matrix. It could be found that the CTEs decreased with the increase of CNTs content. At 300 °C, the CTE of 0.75% CNTs/Al composite was 22.65×10-6 K-1, which was 13.85% and 9.72% lower than that of pure Al and 0.25% CNTs/Al composite, respectively. The improve- ment of dimensional stability of Al matrix could be attributed to the comprehensive effects of low CTE of CNTs, uniform distribution of in-situ synthesized CNTs in Al matrix and good interfacial integrity between the reinforcement and the metal matrix [19].
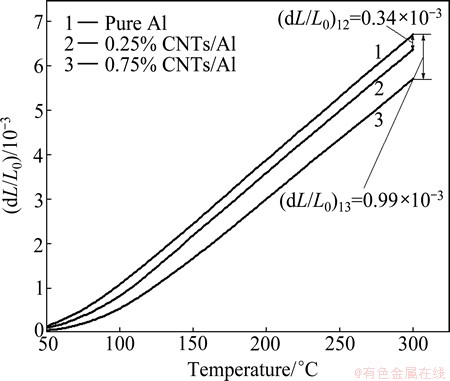
Fig. 2 Thousandth linear change versus temperature curves for pure Al, 0.25% and 0.75% CNTs/Al in-situ composites
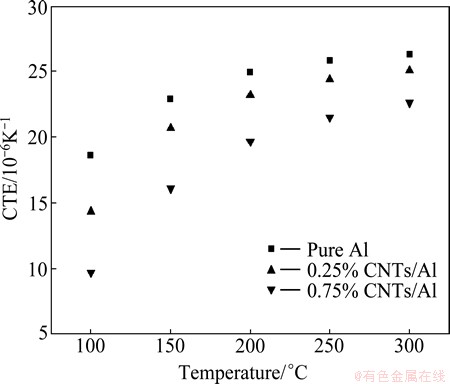
Fig. 3 Variation of coefficient of thermal expansion versus temperature for pure Al, 0.25% and 0.75% CNTs/Al in-situ composites
3.3 Mechanical properties of CNTs/Al composites
Figure 4 shows the hardness and tensile strength of CNTs/Al in-situ composites with different contents of CNTs. It is indicated that the hardness of the composites increased with the rise of CNTs content within 0.75%. When the content of CNTs was increased to 1%, the hardness of the composites decreased rapidly, due to the segregation of CNTs on aluminum grain boundaries and the weakening of the grain bonding strength. The hardness of 0.75% CNTs/Al composites was HV 62.58, which was 35.34% higher than that of pure Al bulk (HV 46.24), revealing that the reinforcement effect of in-situ synthesized CNTs is remarkable. The tensile strength curve of CNTs/Al composites had similar variation trend with the hardness curve. When the content of CNTs was 0.75%, the tensile strength reached the peak value, 184.9 MPa, which was 35.56% higher than that of pure Al bulk. Though the content of CNTs was relatively low, their remarkable roles in strengthening Al matrix could be fully displayed. The remarkable strengthening was caused by the dispersion strengthening of homogeneously dispersed CNTs in Al matrix. Its preconditions should include the perfect structure of the synthesized CNTs, the molecular-level homogenous mixing and the strong interfacial bonding between CNTs and Al [20,21]. By the in-situ synthesis of CNTs in aluminum matrix, these preconditions could be met.
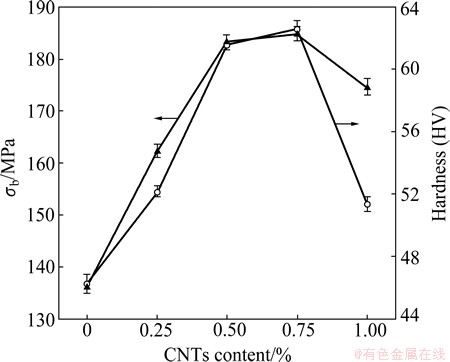
Fig. 4 Hardness and tensile strength of CNTs/Al in-situ composites
To fully understand the strengthening mechanism of CNTs in aluminum matrix composites, the fracture surface and microstructure of the composites were analyzed. Figures 5 and 6 show the SEM image of tensile fracture surface and TEM image of CNTs/Al in-situ composite, respectively. From Fig. 5, it could be observed that the pulled out CNTs homogeneously dispersed on the fracture surface and kept close combination with Al matrix, which could produce effective load translation between the reinforcement and matrix during tensile strain. Thus, the fracture energy and the tensile strength of composites were improved. The interfacial bonding status between CNTs and Al matrix could be known from Fig. 6. In bulk composite, the perfect structure of CNTs was kept. Close interfacial bonding between single CNT and Al matrix was formed, and no reactant such as Al4C3 was found. The CNT stretching across two Al grains could bear stress by sharing a portion of the load and toughen the matrix by a bridging effect [22].

Fig. 5 SEM image of tensile fracture surface of CNTs/Al in-situ composite
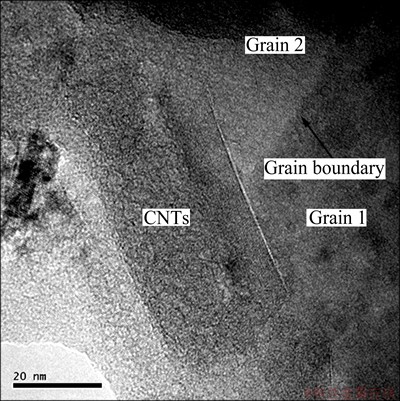
Fig. 6 TEM image of CNTs/Al in-situ composite
Finally, it is worth mentioned that the mechanical properties of CNTs/Al in-situ composites could be further improved by optimizing the structure and dispersion of CNTs grown in aluminum matrix.
4 Conclusions
1) High-quality CNTs were synthesized in Al matrix by in-situ CVD of methane at the temperature (650 °C) near the melting point of Al. Compared with using Fe/Al and Co/Al catalysts, using Ni/Al catalyst, the as-grown CNTs possessed higher yield, ideal degree of graphitization and perfect structure.
2) Using the approach of in-situ technique, relatively homogeneous dispersion of CNTs in Al powders was achieved. The CNTs/Al in-situ composites showed obvious improvements on mechanical properties and dimensional stability, due to the superior dispersion of CNTs in Al matrix and well interfacial bonding between CNTs and Al.
References
[1] IIJIMA S. Helical microtubes of graphitic carbon [J]. Nature, 1991, 354: 56-58.
[2] KANG J L, NASH P, LI J J, SHI C S, ZHAO N Q. Achieving highly dispersed nanofibres at high loading in carbon nanofibre-metal composites [J]. Nanotechnology, 2009, 20: 235607-235613.
[3] BAUHOFER W, KOVACS J Z. A review and analysis of electrical percolation in carbon nanotube polymer composites [J]. Composites Science and Technology, 2009, 69: 1486-1498.
[4] CHO J, BOCCACCINI A R, SHAFFER M S P. Ceramic matrix composites containing carbon nanotubes [J]. Journal of Materials Science, 2009, 44: 1934-1951.
[5] MORSI K, ESAWI A M K, LANKA S, SAYED A, TAHER M. Spark plasma extrusion (SPE) of ball-milled aluminum and carbon nanotube reinforced aluminum composite powders [J]. Composites Part A, 2010, 41: 322-326.
[6] LI Q H, ZHOU Q H, DENG D, YU Q Z, GU L, GONG K D, XU K H. Enhanced thermal and electrical properties of poly (D,L-lactide)/ multi-walled carbon nanotubes composites by in-situ polymerization [J]. Transactions of Nonferrous Metals Society of China, 2013, 23: 1421-1427.
[7] LIM Y J, PARK M Y, LEE S K, LEE W K , JO N J. Polyaniline and multi-walled carbon nanotube composite electrode for rechargeable battery [J]. Transactions of Nonferrous Metals Society of China, 2012, 22: s717-s721.
[8] RUL S, LEFEVRE-SCHLICK F, CAPRIA E, LAURENT C, PEIGNEY A. Percolation of single-walled carbon nanotubes in ceramic matrix nanocomposites [J]. Acta Materialia, 2004, 52: 1061-1067.
[9] BALANI K, ZHANG T, KARAKOTI A, LI W Z, SEAL S, AGARWAL A. In situ carbon nanotube reinforcements in a plasma-sprayed aluminum oxide nanocomposite coating [J]. Acta Materialia, 2008, 56: 571-579.
[10] HE C N, ZHAO N Q, SHI C S, DU X W, LI J J, LI H P, CUI Q R. An approach to obtaining homogeneously dispersed carbon nanotubes in Al powders for preparing reinforced Al-matrix composites [J]. Advanced Materials, 2007, 19: 1128-1132.
[11] KANG J L, LI J J, ZHAO N Q, NASH P, SHI C S, SUN R L. General rules governing carbon nanomaterial growth directly on metal support by chemical vapor deposition [J]. Materials Chemistry and Physics, 2011, 125: 386-389.
[12] KANG J L, LI J J, SHI C S, NASH P, CHEN D J, ZHAO N Q. In situ synthesis of carbon onion/nanotube reinforcements in copper powders [J]. Journal of Alloys and Compounds, 2009, 476: 869-873.
[13] KANG J L, LI J J, ZHAO N Q, NASH P, SHI C S, SUN R L. Study of Mg powder as catalyst carrier for the carbon nanotube growth by CVD [J]. Journal of Nanomaterials, 2011, 2011: 938493-6.
[14] LI H P, KANG J L, HE C N, ZHAO N Q, LIANG C Y, LI B E. Mechanical properties and interfacial analysis of aluminum matrix composites reinforced by carbon nanotubes with diverse structures [J]. Materials Science and Engineering A, 2013, 577:120-124.
[15] DARKEN L S, GURRY R W. Physical chemistry of metals [M]. New York: McGraw Hill Publisher, 1953: 364-366.
[16] CI L J, RYU Z, JIN-PHILLIPP N Y, RUHLE M. Investigation of the interfacial reaction between multi-walled carbon nanotubes and aluminum [J]. Acta Materialia, 2006, 54: 5367-5375.
[17] KIM N S, LEE Y T, PARK J, HAN J B, CHOI Y S, CHOI S Y, CHOO J, LEE G H. Vertically aligned carbon nanotubes grown by pyrolysis of iron, cobalt, and nickel phthalocyanines [J]. Journal of Physical Chemistry B, 2003, 107: 9249-9255.
[18] LEE C J, PARK J, YU J A. Catalyst effect on carbon nanotubes synthesized by thermal chemical vapor deposition [J]. Chemical Physics Letters, 2002, 360: 250-255.
[19] HASSAN S F, GUPAT M. Effect of type of primary processing on the microstructure, CTE and mechanical properties of magnesium/alumina nanocomposites [J]. Composite Structures, 2006, 72: 19-26.
[20] HE P,  X C, LIN T S, LI H X, AN J, MA X, FENG J C, ZHANG Y, LI Q, QIAN Y Y. Improvement of mechanical properties of Sn-58Bi alloy with multi-walled carbon nanotubes [J]. Transactions of Nonferrous Metals Society of China, 2012, 22: s692-s696.
X C, LIN T S, LI H X, AN J, MA X, FENG J C, ZHANG Y, LI Q, QIAN Y Y. Improvement of mechanical properties of Sn-58Bi alloy with multi-walled carbon nanotubes [J]. Transactions of Nonferrous Metals Society of China, 2012, 22: s692-s696.
[21] SUN F J, SHI C S, RHEE K Y, ZHAO N Q. In situ synthesis of CNTs in Mg powder at low temperature for fabricating reinforced Mg composites [J]. Journal of Alloys and Compounds, 2013, 551: 496-501.
[22] LIU S Y, GAO F P, ZHANG Q Y, ZHU X, LI W Z. Fabrication of carbon nanotubes reinforced AZ91D composites by ultrasonic processing [J]. Transactions of Nonferrous Metals Society of China, 2010, 20: 1222-1227.
李海鹏1, 2,范佳薇2,康建立3,赵乃勤1,王雪霞2,李宝娥2
1. 天津大学 材料科学与工程学院,天津 300072;
2. 河北工业大学 材料科学与工程学院,天津 300130;
3. 天津工业大学 材料科学与工程学院,天津 300387
摘 要:采用负载于铝粉上的镍催化剂,成功地在650 °C通过化学气相沉积法在铝基体中原位合成碳纳米管。结构表征表明,所合成的碳纳米管具有较高的石墨化程度和平直的石墨壳层。通过该方法实现铝粉中碳纳米管的弥散分布,其分散效果优于传统机械混合方法。利用所合成的碳纳米管/铝原位复合粉末,采用粉末冶金工艺制备碳纳米管/铝基复合材料。性能测试表明,制备的复合材料的力学性能和尺寸稳定性得到显著提高,其原因在于铝基体中碳纳米管的均匀分散和碳纳米管-铝基体之间良好的界面结合。
关键词:铝基复合材料;碳纳米管;化学气相沉积;原位合成
(Edited by Sai-qian YUAN)
Foundation item: Projects (51071107, 51001080, 51201056) supported by the National Natural Science Foundation of China; Project (2010CB934703) supported by the National Basic Research Program of China; Project (13211027) supported by Science and Technology Plan Project of Hebei Province, China; Project (2011008) supported by Outstanding Youth Science and Technology Innovation Fund of Hebei University of Technology, China
Corresponding author: Nai-qin ZHAO; Tel/Fax: +86-22-27891371; E-mail: nqzhao@tju.edu.cn
DOI: 10.1016/S1003-6326(14)63353-7

