Factors affecting phenol adsorption on clay-solidified grouting curtain
来源期刊:中南大学学报(英文版)2011年第3期
论文作者:陈永贵 叶为民 张可能
文章页码:854 - 858
Key words:clay-solidified grouting curtain; adsorption; phenol; effective factors
Abstract: Batch experiments were conducted to study the adsorption of phenol on clay-solidified grouting curtain (CSGC) and the effects of contact time, pH and adsorbent concentration on the adsorption were investigated. Under the experimental conditions used, 2 d was adequate to determine the equilibrium of phenol adsorption onto CSGC. The amount of phenol adsorbed by CSGC from an initial concentration of 100 mg/L was found to be 8.4 mg/g. The adsorption process includes particle diffusion and liquid film diffusion, and the latter is the predominating step of the adsorption process. The adsorption ability of CSGC decreased with pH but it increased non-linearly with the CSGC concentration. The optimized concentration for CSGC was found to be 20 g/L for the adsorption of 100 mg/L phenol.
J. Cent. South Univ. Technol. (2011) 18: 854-858
DOI: 10.1007/s11771-011-0773-8![]()
CHEN Yong-gui(陈永贵)1, 2, 3, YE Wei-min(叶为民)3, ZHANG Ke-neng(张可能)4
1. State Key Laboratory of Geomechanics and Geotechnical Engineering,
Institute of Rock and Soil Mechanics, Chinese Academy of Sciences, Wuhan 430071, China;
2. School of Civil Engineering and Architecture, Changsha University of Science and Technology,
Changsha 410076, China;
3. Key Laboratory of Geotechnical and Underground Engineering of Ministry of Education,
Tongji University, Shanghai 200092, China;
4. School of Geosciences and Environmental Engineering, Central South University, Changsha 410083, China
? Central South University Press and Springer-Verlag Berlin Heidelberg 2011
Abstract: Batch experiments were conducted to study the adsorption of phenol on clay-solidified grouting curtain (CSGC) and the effects of contact time, pH and adsorbent concentration on the adsorption were investigated. Under the experimental conditions used, 2 d was adequate to determine the equilibrium of phenol adsorption onto CSGC. The amount of phenol adsorbed by CSGC from an initial concentration of 100 mg/L was found to be 8.4 mg/g. The adsorption process includes particle diffusion and liquid film diffusion, and the latter is the predominating step of the adsorption process. The adsorption ability of CSGC decreased with pH but it increased non-linearly with the CSGC concentration. The optimized concentration for CSGC was found to be 20 g/L for the adsorption of 100 mg/L phenol.
Key words: clay-solidified grouting curtain; adsorption; phenol; effective factors
1 Introduction
Phenolic compounds are common contaminants in wastewaters and are generated in the petroleum and petrochemical, coal conversion and phenol-producing industries. Phenols are considered as priority pollutants since they are harmful to organisms at low concentrations and many of them have been classified as hazardous pollutants because of their potential harm to human health [1-3]. The increase in the industrial wastewaters requires that organic compounds including phenol need to be removed more effectively. The removal of phenol from contaminated water can be achieved by several processes such as biodegradation [4], sparging [5], electrochemistry [6], biological technology [7] and oxidation [8]. Most of these processes are ineffective, extremely expensive or generate secondary pollution. In recent years, the adsorption process has received much attention and has become one of the more popular methods for the removal of phenol from wastewater because of its competitive and effective adsorption process. The adsorption of phenol by different adsorbents has been investigated in an attempt to find a relationship between adsorption capacity and adsorbent characteristics such as surface area and pore size distribution for phenol separation in the permissible drinking water concentration range [9-11].
Clay-solidified grouting curtain (CSGC) has been used as an anti-seepage system in landfills to prevent the leachates containing much organic pollutants and heavy metal ions from permeating into surrounding soils and groundwater. The adsorption of some heavy metal ions onto CSGC has been studied by ZHANG et al [12] and CHEN et al [13]. Until now, few reports exist regarding the application of CSGC for the removal of phenol. The aim of this work is to investigate, experimentally, the potential of CSGC to adsorb phenolic pollutants using phenol as a model component. The effects of contact time, pH and adsorbent concentration were studied by batch experiments.
2 Materials and methods
2.1 Adsorbent
The clay-solidified grout consists of a main component, a frame reagent, an additive and a solvent. In this experiment, the main component was clay with an average size of no more than 80 μm from Yichun, China, whose components are described in Table 1. The frame reagent was 32.5 general Portland cement and the additive was water glass with a modulus of 3.2. The clay-solidified grout was mixed according to the pre-determined composition (Table 2) and placed into the model patterns. The samples were left for about 14 d in water and the CSGC samples were thus obtained [13].
Table 1 Components of used clay from Yichun, China (mass fraction, %)
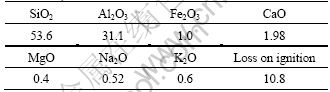
Table 2 Composition of clay-solidified grout

According to the method suggested by ARNOULD et al [14], the CSGC samples were oven-dried at 80 °C and then the experimental samples were obtained after grinding and sieving with 80 mm sieves.
2.2 Experimental process
The batch adsorption experiments were carried out by allowing an accurately weighed amount of CSGC to reach the equilibrium with phenol solutions of known concentrations. Samples of accurately weighed amounts of CSGC powder were placed in a 200 mL conical flask together with 50 mL of phenol solution of known concentration. The pH was adjusted to the desired value by the addition of dilute HCl or NaOH solution. These solutions were stirred in mechanical shakers until they reached the adsorption equilibrium or for an initialization contact time. The solution was crossed for 24 h and the CSGC was separated by centrifugation for 30 min at a rate of 4 000 r/min. At the end of the equilibrium period, the contents of the bottles were filtered and the supernatant was subsequently analyzed for the residual phenol. The phenol was kept in dark brown bottles to prevent photooxidation.
2.3 Analysis of phenol
With water as the solvent, the concentration of phenol was determined by the method of GALES and BOOTH [15] and this method consists of a spectrophotometric analysis of the developed color resulting from the reaction of phenol with 4-aminoantipyrine [1, 15-16].
The concentration of phenol in the aqueous solution was determined using a double beam UV spectro- photometer (Shimadze UV-9100, Japan) at a wavelength of 270 nm. Before the analysis, a reproducible and linear technical calibration curve was obtained over the concentration range used in this work [15].
The amount of phenol adsorbed on CSGC was calculated from the difference between the initial concentration and the equilibrium concentration in the liquid phase using the following equation:
![]() (1)
(1)
The adsorption was calculated using the following equation:
A=![]() (2)
(2)
where Qe is the equilibrium content of phenol on the adsorbent, mg/g; c0 is the initial concentration of phenol in the suspension, mg/L; ce is the equilibrium concentration of phenol in the supernatant after centrifugation, mg/L; m is the mass of adsorbent, g; and V is the volume of the phenol solution, L.
All the experimental data are the averages of duplicate or triplicate determinations. The relative errors of the data are about 5%.
3 Results and discussion
3.1 Effect of contact time
One gram of CSGC powder was weighed accurately. To comply with the experimental conditions of c0= 100 mg/L, pH=6.0 and room temperature, a part of the clear upper layer solution was removed at regular intervals for a concentration determination until equilibrium was reached. According to Eq.(1), the adsorption capacity of CSGC can be determined. After the remainder was kept constant and the volume was corrected, the data were obtained, as shown in Fig.1. These results show that the adsorption of phenol on CSGC occurs quickly and 2 d is adequate to obtain adsorption equilibrium under the chosen experimental conditions. The remaining phenol concentration becomes asymptotic over time after 2 d of shaking. After 2 d contact time, the removal of phenol from the solution by CSGC is constant with increasing the contact time. Therefore, in the following experiments, a time period of 2 d was selected for the determination of phenol equilibrium adsorption on CSGC. The amount of phenol adsorbed by CSGC from an initial concentration of 100 mg/L was 8.4 mg/g.
The kinetic results shown in Fig.1 can be used to determine if the particle diffusion is a rate-limiting step for the phenol adsorption onto CSGC. WEBER and MORRIS [17] reported that if the particle diffusion is involved in the sorption process then a plot of adsorbate uptake versus the square root of time would result in a linear relationship and that the particle diffusion would be the rate-controlling step if this line passes through the origin. As shown in Fig.2, the results can be represented by such a linear relationship but they do not pass through the origin. This indicates that the particle diffusion is involved in the sorption process but it is not the only rate-limiting mechanism and that some other mechanisms are involved as well [1, 17].
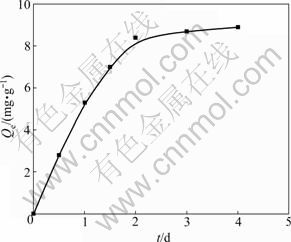
Fig.1 Dynamics of phenol uptake by CSGC
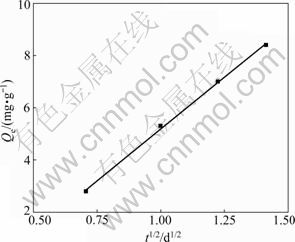
Fig.2 Plot of phenol adsorption versus square root of time for CSGC
According to Ref.[18], the adsorption rate constant k can be calculated using the following two equations, respectively:
-ln(1-F)=kt (3)
F=Qt/Q∞ (4)
where Qt and Q∞ are the adsorption amounts at time t and at equilibrium, respectively. A straight line is obtained by plotting -ln(1-F) versus t, as shown in Fig.3. The adsorption rate constant of CSGC can be determined from the slope of the straight line, and k=1.35 d-1. The linear relationship of -ln(1-F) vs t indicates that the liquid film diffusion is the predominant step during the adsorption process.

Fig.3 Determination of adsorption rate constant of phenol adsorption by CSGC
3.2 Effect of pH on cadmium adsorption
The adsorption of phenol by CSGC was studied at various pH values and at room temperature. Phenol solutions of 100 mg/L were prepared and adjusted to pH values of 2.0, 3.5, 6.0 and 7.5. CSGC was added at 2.0 g/L and adsorption was carried out until equilibrium. The results are shown in Fig.4.

Fig.4 Effect of pH on adsorption of phenol on CSGC
The amount of adsorbed phenol decreases with increasing solution pH, as shown in Fig.4. This can be attributed to the pH dependence of phenol ionization. The ionic fraction of the phenolate ion (φions) can be calculated using the following equation [19]:
![]() (5)
(5)
Obviously, φions increases as the pH value increases. Phenol as a weak acid (pKa=9.96) will undergo less adsorption at higher pH values because of the repulsive forces prevailing at higher pH values. Similar behavior has been reported by TORA et al [20] for the adsorption of phenol by red mud.
The phenol solution is weakly acidic after phenol dissolution but the phenolate ion is more soluble when the pH value is higher than 7. Only when the chemical bonds between the strong adsorbate and water is broken, adsorption can take place. In addition, the adsorption force will be decreased as the repulsion between the adsorbent surface and the dissociated adsorbate increases. Under the experimental conditions used, the amount of adsorbed phenol on CSGC is 9.6-10.6 mg/g and the adsorption capacity increases as the pH value decreases. The ionization coefficient (pKa) of phenol is 9.96. At a pH value less than 9.96, phenol exists in a neutral form as C6H5OH, and in solutions of pH value of 2.0-7.5 [21], it is neutral. The effective adsorption location changes little within the experimental pH range, so the amount of adsorbed phenol on CSGC does not change significantly.
3.3 Effect of CSGC concentration
Under the experimental conditions of c0=100 mg/L, pH=6.0 and room temperature, the average removal was calculated for each adsorbent concentration and the change in percentage removal is shown in Fig.5 as a function of adsorbent concentration. Although the adsorbed amount increases with the adsorbent concentration, this increase is not linear. At a certain adsorbent concentration, the removal percentage does not increase further and remains constant as the CSGC concentration is increased. This confirms the results obtained by AL-MALAH et al [22] and AL-ASHEH et al [23] for bentonite. According to our experimental results, the optimized concentration is 20 g/L for CSGC for the total adsorption of 100 mg/L phenol.

Fig.5 Change in adsorption with adsorbent concentration
4 Conclusions
1) Phenol was largely removed by CSGC from an aqueous solution. The adsorption decreased at a pH value between 2 and 7.5. A non-linear increase in adsorption with CSGC concentration was observed.
2) The adsorption process includes the particle diffusion and the liquid film diffusion, and the latter is the dominant step during the adsorption process.
3) Raw materials for the production of CSGC are cheap and easily available in China. The data obtained may be useful for the design of a cheap treatment process using CSGC for the removal of phenol from landfill leachate or from dilute industrial effluents.
References
[1] BANAT F A, AL-BASHIR B, AL-ASHEH S, HAYAJNEH O. Adsorption of phenol by bentonite [J]. Environmental Pollution, 2000, 107(3): 391-398.
[2] STEEVENSZ A, AL-ANSARI M M, TAYLOR K E, BEWTRA J K, BISWAS N. Comparison of soybean peroxidase with laccase in the removal of phenol from synthetic and refinery wastewater samples [J]. Journal of Chemical Technology and Biotechnology, 2009, 84(5): 761-769.
[3] RICHARDS S, BOUAZZA A. Phenol adsorption in organo-modified basaltic clay and bentonite [J]. Applied Clay Science, 2007, 37(1/2): 133-142.
[4] ARVIN E, JENSEN B K, GUNDERSEN A T. Biodegradation kinetics of phenols in an aerobic biofilm at low concentrations [J]. Water Science and Technology, 1991, 23(7/8/9): 1375-1384.
[5] TSAI Y J. Air flow paths and porosity/permeability change in a saturated zone during in situ air sparging [J]. Journal of Hazardous Materials, 2007, 142(1/2): 315-323.
[6] ABDELWAHAB O, AMIN N K, EL-ASHTOUKHY E-S Z. Electrochemical removal of phenol from oil refinery wastewater [J]. Journal of Hazardous Materials, 2009, 163(2/3): 711-716.
[7] GHOLAMREZA M, MARYAM M, BEHNAM B. Biological removal of phenol from strong wastewaters using a novel MSBR [J]. Water Research, 2009, 43(5): 1295-1302.
[8] KALLEL M, BELAID C, MECHICHI T, KSIBI M, ELLEUCH B. Removal of organic load and phenolic compounds from olive mill wastewater by Fenton oxidation with zero-valent iron [J]. Chemical Engineering Journal, 2009, 150(2/3): 391-395.
[9] ROOSTAEI N, HANDAN T F. Removal of phenol from aqueous solutions by adsorption [J]. Journal of Environmental Management, 2004, 70(2): 157-164.
[10] CABAL B, TSYNTSARSKI B, BUDINOVA T, PETROV N, PARRA J B, ANIA C O. Improved phenol adsorption on carbons after mild temperature steam reactivation [J]. Journal of Hazardous Materials, 2009, 166(2/3): 1289-1295.
[11] SANDRO F, RAQUEL F M, WILLIAN F, MARCELO R E. Water remediation by adsorption of phenol onto hydrophobic modified clay [J]. Water, Air, and Soil Pollution, 2009, 199(1/4): 107-113.
[12] ZHANG Ke-neng, CHEN Yong-gui, DENG Fei-yue, TIAN Qing-yu. Retention of clay-solidified grouting curtain to Cd2+, Pb2+, Hg2+ in landfill of municipal solid waste [J]. Journal of Central South University of Technology, 2004, 11(4): 419-422.
[13] CHEN Yong-gui, ZHANG Ke-neng, ZOU Yin-sheng, DENG Fei-yue. Removal of Pb2+ and Cd2+ by adsorption on clay-solidified grouting curtain for waste landfills [J]. Journal of Central South University of Technology, 2006, 13(2): 166-170.
[14] ARNOULD M, SCHROEDER P R., MYERS T E. Predictive hydrologic model for contaminant leaching and liner effectiveness at dredged material confined disposal facilities [C]// CLARKE MCNAIR E. Proceedings of the 2nd International Conference on Dredging and Dredged Material Placement. Orlando: ASCE, 1994: 1507-1516.
[15] GALES M E, BOOTH R L. Automated 4AAP phenolic method [M]. Washington D C: Environmental Protection Agency, 1976: 540.
[16] MOHD D A T, HAMEED B H, AHMAD A L. Batch adsorption of phenol onto physiochemical-activated coconut shell [J]. Journal of Hazardous Materials, 2009, 161(2/3): 1522-1529.
[17] WEBER W J, MORRIS J C. Kinetics of adsorption on carbon from solution [J]. Journal Sanitary Engineering Division, American Society of Chemical Engineering, 1963, 89: 31-59.
[18] ZHANG Shu-qin, HOU Wan-guo. Adsorption behavior of Pb2+ on montmorillonite [J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2008, 320(1/3): 92-97.
[19] CALACE N, NARDI E, PETRONIO B M, PIETROLETTI M. Adsorption of phenols by papermill sludges [J]. Environmental Pollution, 2002, 118(3): 315-319.
[20] TORA A, CENGELOGLUB Y, AYDINA M E, ERSOZ M. Removal of phenol from aqueous phase by using neutralized red mud [J]. Journal of Colloid and Interface Science, 2006, 300(2): 498-503.
[21] JIANG Zhan-peng, YANG Jin-xiang. The competitive adsorption of phenol and heavy metals onto activated carbon [J]. Technology of Water Treatment, 1989, 5(6): 313-319. (in Chinese)
[22] AL-MALAH K, AZZAM M O J, ABU-LAIL N I. Olive mills effluent (OME) wastewater post-treatment using activated clay [J]. Separation and Purification Technology, 2000, 20(2/3): 225-234.
[23] AL-ASHEH S, BANAT F, ABU-AITAH L. Adsorption of phenol using different types of activated bentonites [J]. Separation and Purification Technology, 2003, 33(1): 1-10.
(Edited by PENG Chao-qun)
Foundation item: Project(40802064) supported by the National Natural Science Foundation of China; Project(Z110805) supported by the Open Research Fund of State Key Laboratory of Geomechanics and Geotechnical Engineering, Institute of Rock and Soil Mechanics, Chinese Academy of Sciences; Project(200925) supported by the Progress and Innovation Fund for the Transportation Science and Technology of Hunan Province, China
Received date: 2010-01-21; Accepted date: 2010-09-27
Corresponding author: CHEN Yong-gui, Associate professor, PhD; Tel: +86-21-65982384; E-mail: yg-chen@163.com

