砷、锑和铋对铜电沉积及阳极氧化机理的影响
肖发新1, 2,郑雅杰1,简洪生1,龚竹青1,许 卫1
(1. 中南大学 冶金科学与工程学院,湖南 长沙,410083;
2. 河南科技大学 材料科学与工程学院,河南 洛阳,471003)
摘 要:采用循环伏安及交流阻抗研究As(Ⅲ,Ⅴ),Sb(Ⅲ,Ⅴ)和Bi(Ⅲ)对铜电沉积及阳极氧化机理的影响。研究结果表明:电解底液循环伏安分别在0.06 V和-0.25 V出现还原峰a和b,在0.10 V和0.23 V出现氧化峰b′和a′。 As(Ⅲ)加速铜的电沉积,As(Ⅴ),Sb(Ⅲ,Ⅴ)和Bi(Ⅲ)均改变铜的沉积机理,使两步反应变成一步反应,其中,加入Sb(Ⅲ,Ⅴ)和Bi(Ⅲ)的电解液在-0.13 V附近出现杂质Sb和Bi的还原峰;这些杂质均抑制铜的氧化反应,改变阳极氧化机理,使两步氧化变为一步氧化反应;将As(Ⅲ,Ⅴ),Sb(Ⅲ,Ⅴ)和Bi(Ⅲ)单独加入电解液中,电极过程均产生电活性物质吸附,均使电极过程阻抗减小。
关键词:砷;锑;铋;铜电沉积;阳极氧化
中图分类号:TF804.8;TF811;TF111.52 文献标识码:A 文章编号:1672-7207(2009)03-0575-06
Influence of As, Sb and Bi on electrodepositing and anode oxidation mechanism of copper
XIAO Fa-xin1, 2, ZHENG Ya-jie1, JIAN Hong-sheng1, GONG Zhu-qing1, XU Wei1
(1. School of Metallurgical Science and Engineering, Central South University, Changsha, 410083, China;
2. School of Materials Science and Engineering, Henan University of Science and Technology, Luoyang 471003, China)
Abstract: The influences of As(Ⅲ,Ⅴ), Sb(Ⅲ,Ⅴ), Bi(Ⅲ) on the electrodepositing and anode oxidation mechanism of copper were studied using cyclic voltammetric and AC impedance techniques. The results show that the reduction peaks of a and b occur at the cathodic potential of 0.06 V(a) and -0.25 V(b) in the reduction process of base electrolyte, and the corresponding oxidation peaks occur at the anodic potential of 0.23 V(a′) and 0.10 V(b′), respectively. The As(Ⅲ) ion promotes the electrodepositing of copper. The electrodepositing process of copper is changed to single-step reaction after As(Ⅴ), Sb(Ⅲ,Ⅴ) and Bi(Ⅲ) are added. The reduction peak of impurity occurs at -0.13 V approximately after Sb(Ⅲ,Ⅴ) and Bi(Ⅲ) are added. After these impurities are added, the anode oxidation reaction of copper is restrained and the oxidation process is changed to singe-step reaction. The electroactive species are adsorbed to electrode surface during the electrodepositing process after theses impurities are added, which decreases the impedance of electrodepositing process.
Key words: As; Sb; Bi; electrodepositing of copper; anode oxidation
随着我国铜冶炼的迅猛发展,铜精矿资源日趋紧 缺[1-2],其杂质含量显著提高[3-4],尤其是砷、锑、铋在电解液的积累因矿石品位下降而日益下降[5-7]。这些杂质电极电位与铜的电极电位接近,易与铜发生共沉积,影响铜的电沉积和阳极铜的氧化过程[8-10]。因此,研究砷、锑、铋在铜电沉积中的电化学行为非常必要。目前,关于这方面的研究较多,但大部分研究仅限于As(Ⅴ),Sb(Ⅲ)和Bi(Ⅲ)[11-15],而就As(Ⅲ)和Sb(Ⅴ)对铜电沉积影响的研究较少。这些研究中As质量浓度仅为1~4 g/L[11-13],目前铜电解液中As质量浓度高达10 g/L以上,因而,上述研究不能反映高砷铜电解机理。此外,这些研究均很少涉及杂质对阳极氧化的影响。为此,本文作者采用循环伏安及交流阻抗研究As(Ⅲ, Ⅴ),Sb(Ⅲ, Ⅴ)和Bi(Ⅲ)对铜阴极沉积及阳极氧化机理的影响。
1 实 验
采用(45 g/L Cu2+和185 g/L H2SO4)硫酸铜和硫酸配置成电解底液;采用亚砷酸铜(自制)、砷酸铜、三氧化二锑、双氧水、硫酸铋、盐酸配置高浓度的As(Ⅲ, Ⅴ),Sb(Ⅲ, Ⅴ)和Bi(Ⅲ)溶液,将这些溶液加入到电解底液中,并调整溶液硫酸浓度和铜离子质量浓度分别为185 g/L 和45 g/L;所有溶液均采用分析纯试剂及二次蒸馏水配制。
采用电化学工作站CHI600A(上海辰华仪器公司)进行电化学测试,测试采用三电极体系,研究电极为铜电极(直径为1 mm),辅助电极为大面积铂电极,参比电极为饱和甘汞电极,采用带有Luggin毛细管的盐桥以消除不同溶液间的液体接界电位,实验温度为 65 ℃,交流阻抗实验频率为0.1~1×105 Hz。
2 结果与讨论
2.1 As(Ⅲ)对铜电沉积的影响
在扫描速度为10 mV/s,电极电位为0.8~-0.6 V范围内测量电解底液的循环伏安曲线,结果如图1所示。由图1可知,电解底液循环伏安阴极分支上分别在0.06 V和-0.25 V附近出现还原峰a和b,分别对应铜离子的第1步和第2步还原反应;在电位回扫过程中,分别出现氧化峰b′和a′,峰电位分别约为0.10 V和0.23 V,对应着铜的第1步和第2步氧化反应。a,b,a′和b′对应的反应式分别为:

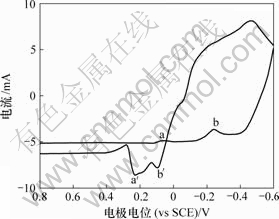
图1 电解底液循环伏安曲线
Fig.1 Cyclic voltammetric curves of base electrolyte
在相同条件下测定含As(Ⅲ)的电解液循环伏安曲线。在完整曲线中,由于阴极(阳极)峰几乎重叠,不利于比较,因此,选取曲线的一部分观察阴极峰以及阳极峰的变化情况。As(Ⅲ)对铜电沉积影响及对阳极氧化的影响如图2和图3所示。
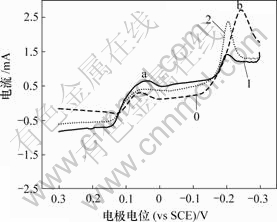
0—底液;1—底液+2 g/L As(Ⅲ);2—底液+15 g/L As(Ⅲ)
图2 As(Ⅲ)对铜电沉积循环伏安曲线阴极分支的影响
Fig.2 Influence of As(Ⅲ) on electrodepositing of copper
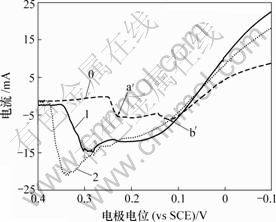
图3 As(Ⅲ)对铜电沉积循环伏安曲线阳极分支的影响
Fig.3 Influence of As(Ⅲ) on anode oxidation of copper
由图2可知,加入As(Ⅲ)后,阴极还原峰a电流增大,还原峰b电位正移。由图3可知,阳极过程在0.32 V附近出现氧化峰c′,峰电流显著大于氧化峰a′和b′电流。
在电解液中加入As(Ⅲ)后,As(Ⅲ)在电极表面吸附,形成“离子桥”,促进铜的电沉积,从而使还原峰a电流增大,还原峰b电位正移。在电位回扫过程 中,由于As(Ⅲ)在电极表面吸附,抑制铜的电化学溶解,改变阳极氧化机理,使两步氧化变为一步氧化反应,由于阳极过电位显著增大,氧化峰c′电流急剧增大。此外,随着电极电位正移,Girgis等[15]认为,在含2 g/L As(Ⅲ)的铜电解液循环伏安阳极分支上,当电位大于0.25 V时,还发生As(Ⅲ)的氧化反应,其反应如下:
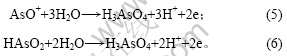
上述反应也使氧化峰c′电流增大。当电极电位为0.05 V,温度为65 ℃时,测得加入As(Ⅲ)后铜电沉积交流阻抗图谱如图4所示。
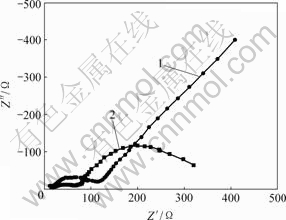
1—底液;2—底液+10 g/L As(Ⅲ)
图4 As(Ⅲ)对铜电沉积交流阻抗图谱的影响
Fig.4 Influences of As(Ⅲ) on impedance complex plane of copper electrodepositing
由图4可知,不加As(Ⅲ)时,电极阻抗图谱为感抗弧加直线。这是典型的简单电荷传递反应,电极过程受浓差极化和电化学极化混合控制,其等效电路由电化学反应电阻Rt,反应物O的浓差电阻RWO和浓差电容CWO,产物R的浓差电阻RWR和浓差电容CWR串联组成,整个电极的等效电路如图5所示。
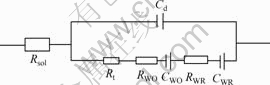
1—电解底液;2—电解底液+10 g/L As(Ⅲ)
图5 简单电荷传递反应电极等效电路
Fig.5 Impedance equivalent circuit of consecutive charge-transfer reaction
加入As(Ⅲ)后,电极阻抗图谱为2个感抗弧,且第1个感抗弧直径减小,这是由电化学吸附所致,其对应的电极等效电路如图6所示。
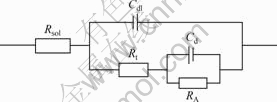
图6 具有电化学吸附电极阻抗等效电路
Fig.6 Impedance equivalent circuit of electrode with adsorption of electroactive species
可见,加入As(Ⅲ)后,电极过程发生电活性性物质吸附,加速铜电沉积,电极反应阻抗减小,感抗弧直径减小,这与循环伏安在该电位下峰电流增大结果一致。
2.2 As(Ⅴ)对铜电沉积的影响
当扫描速度为10 mV/s时,As(Ⅴ)对铜电沉积及阳极氧化影响如图7和图8所示。
0—底液;1—底液+2 g/L As(Ⅴ);2—底液+15 g/L As(Ⅴ)
图7 As(Ⅴ)对铜电沉积循环伏安曲线阴极分支的影响
Fig.7 Influence of As(Ⅴ) on electrodepositing of copper
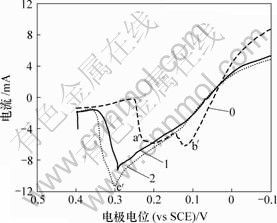
0—底液;1—底液+2 g/L As(Ⅴ);2—底液+15 g/L As(Ⅴ)
图8 As(Ⅴ)对铜电沉积循环伏安曲线阳极分支的影响
Fig.8 Influence of As(Ⅴ) on anode oxidation of copper
由图7可知,加入As(Ⅴ)后,阴极过程在0.04 V附近出现新的还原峰c,其峰电流显著大于还原峰a和还原峰b电流。由图8可知,阳极过程在0.30 V附近出现氧化峰c′。c和c′对应的反应式如下:
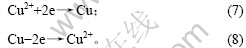
在电解液中加入As(Ⅴ)后,随着电极电位的负移,电极表面除发生铜的沉积反应外,还发生以下反应:
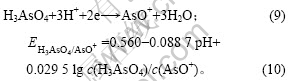
将c(H3AsO4)=0.2 mol/L,pH=-0.28代入式(10)
可得: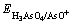 =0.662 V,大于铜的析出电位
=0.662 V,大于铜的析出电位
(0.334 V)。因此,当As(Ⅴ)加入至铜电解液中时,首先发生As(Ⅴ)的还原反应,其次发生铜的沉积反应。加入As(Ⅴ)后,As(Ⅴ)吸附在电极表面,改变铜的电沉积机理,使两步反应变成一步反应。因此,峰电流增大,反应(9)发生的可能性也使之增大。
在电位回扫过程中,由于As(Ⅴ)在电极表面吸附,抑制铜的电化学溶解,改变阳极氧化机理,使两步氧化变为一步氧化反应。
在相同条件下测试了含10 g/L As(Ⅴ)电解液交流阻抗图谱,所得结果与图4相似。电极阻抗图谱由感抗弧加直线变成2个感抗弧,且第1个感抗弧直径增大。不加As(Ⅴ)时,还原过程无电活性物质被吸附。加入As(Ⅴ)后,电极过程中电活性物质被吸附,使电极阻抗减小,感抗弧直径减小。
2.3 Sb(Ⅲ)对铜电沉积的影响
当扫描速度为10 mV/s时,Sb(Ⅲ)对铜电沉积及阳极氧化影响如图9和图10所示。
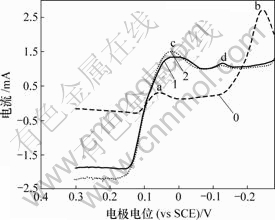
0—底液;1—底液+0.2 g/L Sb(Ⅲ);2—底液+0.6 g/L Sb(Ⅲ)
图9 Sb(Ⅲ)对铜电沉积循环伏安曲线阴极分支的影响
Fig.9 Influence of Sb(Ⅲ) on electrodepositing of copper
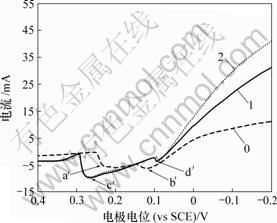
0—底液;1—底液+0.2 g/L Sb(Ⅲ);2—底液+0.6 g/L Sb(Ⅲ)
图10 Sb(Ⅲ)对铜电沉积循环伏安曲线阳极分支的影响
Fig.10 Influence of Sb(Ⅲ) on anode oxidation of copper
由图9可知,加入Sb(Ⅲ)后,阴极过程在0.04 V附近出现还原峰c,在-0.13 V附近出现还原峰d。由图10可知,阳极过程在0.28 V附近出现氧化峰c′,在0.09 V附近出现氧化峰d′。
加入Sb(Ⅲ)后,Sb(Ⅲ)吸附在电极表面,改变铜的电沉积机理,使两步反应变成一步反应。随着电极电位的负移,电极表面除了发生铜的沉积反应外,还发生以下反应:
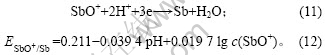
将c(SbO+)=0.008 2 mol/L,pH=-0.28代入式(12)
可得: =0.181,低于铜的析出电位(0.334 V)。
=0.181,低于铜的析出电位(0.334 V)。
鲁道荣等[13]对含0.2~0.8 g/L Sb(Ⅲ)的铜电解液在 1.5 kA/m2时电沉积所得沉积层进行电子能谱分析,发现沉积层中含有Sb。因此,还原峰d为杂质Sb的还原峰,反应式如(11)所示。
在电位回扫过程中,由于Sb(Ⅲ)在电极表面的吸附,抑制铜的氧化反应均有影响,改变阳极氧化机理,使两步氧化变为一步氧化反应。除铜的氧化外,电极表面还发生锑的氧化反应,因此,氧化峰d′为还原峰d对应的阳极氧化峰。
在相同条件下测试了含0.6 g/L Sb(Ⅲ)电解液交流阻抗图谱,所得结果与图4相似。电极阻抗图谱由感抗弧加直线变成2个感抗弧,且第1个感抗弧直径增大。不加Sb(Ⅲ)时,还原过程无电活性物质被吸附。加入Sb(Ⅲ)后,电极过程电活性物质被吸附,使电极阻抗减小,感抗弧直径减小。
2.4 Sb(Ⅴ)对铜电沉积的影响
当扫描速度为10 mV/s时Sb(Ⅴ)对铜电沉积及阳极氧化影响与图9和图10相似。加入Sb(Ⅴ)后,阴极过程在0.04 V附近出现还原峰c;阳极过程在0.28 V附近出现氧化峰c’。当Sb(Ⅴ)质量浓度达到0.6 g/L以上时,在-0.13 V附近出现还原峰d,在0.09 V附近出现氧化峰d′。
加入Sb(Ⅴ)后,Sb(Ⅴ)离子吸附在电极表面,改变铜的电沉积机理,使两步反应变成一步反应。随着电极电位的负移,电极表面除了发生铜的沉积反应外,还发生以下反应:
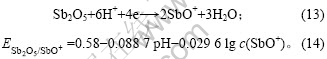
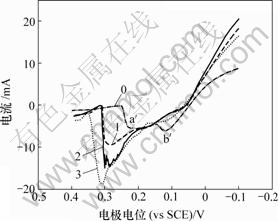
将c(SbO+) = 10-5 mol/L和pH= -0.28代入式(14),
得到: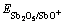 =0.753 V,这显著高于铜的析出电
=0.753 V,这显著高于铜的析出电
位(0.334 V)。因此,当Sb(Ⅴ)加入至铜电解液中时,体系首先发生Sb (V)的还原反应,其次发生铜的沉积反应。根据上述分析,当Sb(Ⅴ)浓度较大时,反应(13)生成的SbO+继续在-0.13 V附近发生锑的沉积反应,出现还原峰d。
在电位回扫过程中,由于Sb(Ⅴ)在电极表面的吸附,抑制铜的氧化反应,改变阳极氧化机理,使两步氧化反应变为一步氧化反应。除铜的氧化外,电极表面发生锑的氧化反应,出现还原峰d对应的阳极氧化峰d′。
在相同条件下测试了含0.6 g/L Sb(Ⅴ)电解液交流阻抗图谱,所得结果与图4相似。电极阻抗图谱由感抗弧加直线变成2个感抗弧,且第1个感抗弧直径增大。不加Sb(Ⅴ)时,还原过程无电活性物质被吸附。加入Sb(Ⅴ)后,电极过程电活性物质被吸附,使电极阻抗减小,感抗弧直径减小。
2.5 Bi(Ⅲ)对铜电沉积的影响
当扫描速度为10 mV/s时测得Bi(Ⅲ)对铜电沉积循环伏安阴极分支和阳极分支影响如图11和图12 所示。
0—底液;1—底液+0.25 g/L Bi(Ⅲ);2—底液+0.5 g/L Bi(Ⅲ);3—底液+0.8 g/L Bi(Ⅲ)
图11 Bi(Ⅲ)对铜电沉积循环伏安曲线阴极分支的影响图
Fig.11 Influence of Bi(Ⅲ) on electrodepositing of copper
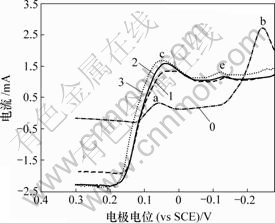
0—底液;1—底液+0.25 g/L Bi(Ⅲ);2—底液+0.5 g/L Bi(Ⅲ);3—底液+0.8 g/L Bi(Ⅲ)
图12 Bi (Ⅲ)对铜电沉积循环伏安曲线阳极分支的影响
Fig.12 Influence of Bi(Ⅲ) on anode oxidation of copper
由图11可知,加入Bi(Ⅲ)后,阴极过程在0.04 V附近出现还原峰c,在电极电位为-0.12 V附近出现新的还原峰e。由图12可知,阳极过程在0.3 V出现氧化峰c′。
电解液中加入Bi(Ⅲ)后,Bi(Ⅲ)吸附在电极表面,改变铜的电沉积机理,使两步反应变成一步反应。随着电极电位的负移,电极表面除了发生铜的沉积反应外,还发生以下反应:

将c(BiO+) = 0.003 8 mol/L和pH = -0.28代入式
(16)可得: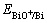 = 0.283 V,略低于铜的析出电位
= 0.283 V,略低于铜的析出电位
(0.334 V)。鲁道荣等[13]对含0.1~1.0 g/L Bi(Ⅲ)的铜电解液在1.5 kA/m2下电沉积所得沉积层进行电子能谱分析,发现沉积层中含有Bi,因此,还原峰e为杂质Bi的还原峰。
在电位回扫过程中,由于Bi(Ⅲ)在电极表面吸附,抑制铜的氧化反应,改变阳极氧化机理,使两步氧化变为一步氧化反应。
在相同条件下测试了含0.25 g/L Bi(Ⅲ)电解液交流阻抗图谱,所得结果与图4相似。电极阻抗图谱由感抗弧加直线变成2个感抗弧,且第1个感抗弧直径增大。不加Bi(Ⅲ)时,还原过程无电活性物质被吸附。加入Bi(Ⅲ)后,电极过程电活性物质被吸附,使电极阻抗减小,感抗弧直径减小。
3 结 论
a. 电解底液循环伏安阴极分支上分别在0.06 V和-0.25 V附近出现还原峰a和b,分别对应铜离子的第1步和第2步还原反应;阳极分支上分别在0.10 V和0.23 V附近出现氧化峰b′和a′,分别对应着铜的第1步和第2步氧化反应。
b. As(Ⅲ)加速铜的电沉积,使还原峰a电流增大,还原峰b电位正移。As(Ⅴ),Sb(Ⅲ, Ⅴ)和Bi(Ⅲ)均改变铜的沉积机理,使两步反应变成一步反应,其中,加入Sb(Ⅲ, Ⅴ)和Bi(Ⅲ)的电解液阴极还原过程在-0.13 V附近出现杂质Sb和Bi的还原峰。这些杂质均抑制铜的氧化反应,改变阳极氧化机理,使两步氧化变为一步氧化反应。
c. As(Ⅲ, Ⅴ),Sb(Ⅲ, Ⅴ)和Bi(Ⅲ)单独加入电解液中,电极过程均电活性物质被吸附,均使电极过程阻抗减小。
参考文献:
[1] ZHENG Ya-jie, XIAO Fa-xin, WANG Yong, et al. Industrial experiment of copper electrolyte purification by copper arsenite[J]. Journal of Central South University of Technology, 2008,15(2): 204-208.
[2] XIAO Fa-xin, ZHENG Ya-jie, XU Wei, et al. Novel technology of purification of copper electrolyte[J]. Transaction of Nonferrous Metals Society of China, 2007, 17(5): 1069-1074.
[3] XIAO Fa-xin, ZHENG Ya-jie, JIAN Hong-sheng. Preparation technique of copper arsenite and its application in purification of copper electrolyte[J]. Transaction of Nonferrous Metals Society of China, 2008,18(2): 342-346.
[4] WANG Xue-wen, CHEN Qi-yuan, YIN Zhou-lan, et al. Removal of impurities from copper electrolyte with adsorbent containing antimony[J]. Hydrometallurgy, 2003, 69(1/3): 39-44.
[5] Navarro P, Alguacil F J. Adsorption of antimony and arsenic from a copper electrorefining solution onto activated carbon[J]. Hydrometallurgy, 2002, 66(1/3): 101-105.
[6] 仇勇海, 陈白珍, 梅显芝. 控制阴极电势电积法新工艺及其应用[J]. 中南工业大学学报: 自然科学版, 1999, 30(5): 501-504.
QIU Yong-hai, CHEN Bai-zhen, MEI Xian-zhi. Study and application of new technology of controlling potential potential electrowinning[J]. Journal of Central South University of Technology: Natural Science, 1999, 30(5): 501-504.
[7] Cifuentes L, Grisostomo G,ibanez J P, et al. On the electrodialysis of aqueous H2SO4-CuSO4 electrolytes with metallic impurities[J]. Journal of Membrane Science, 2002, 207(1): 1-16.
[8] Ilkhchi M O, Yoozbashizadeh H, Safarzadeh H Y. The effect of additives on anode passivation in electrorefining of copper[J]. Chemical Engineering and Processing, 2007, 46(8): 757-763.
[9] WANG Xue-wen, CHEN Qi-yuan, YIN Zhou-lan, et al. Identification of arsenato antimonates in copper anode slimes[J]. Hydrometallurgy, 2006, 84(3): 211-217.
[10] Lin H K. Extraction of antimony by a copper chloride extractant[J]. Hydrometallurgy, 2004, 73(3): 283-291.
[11] 鲁道荣, 林建新. As5+, Sb3+, Bi3+对阴极铜沉积反应动力学的影响[J]. 合肥工业大学学报, 1997, 20(5): 51-56.
LU Dao-rong, LIN Jian-xin. The effect of As5+, Sb3+, Bi3+ on the kinetics of cathodic copper deposition reaction[J]. Journal of Hefei University of Technology, 1997, 20(5): 51-56.
[12] 鲁道荣. 杂质在铜电解精炼中的电化学行为[J]. 有色金属, 2002, 54(4): 51-53.
LU Dao-rong. Electrochemical behaviors of impurity ions in copper electrorefining[J]. Nonferrous Metals, 2002, 54(4): 51-53.
[13] 鲁道荣, 李学良, 林建新. 砷锑铋对阴极铜沉积过程的影响[J]. 应用化学, 1998, 15(2): 56-59.
LU Dao-rong, LI Xue-liang, LIN Jian-xin. The effects of As, Sb and Bi on the cathodic copper deposition[J]. Chinese Journal of Applied Chemistry, 1998, 15(2): 56-59.
[14] 鲁道荣, 林建新. 铋对铜电解精炼中阴极铜沉积过程的影响[J]. 合肥工业大学学报, 1996, 19(S1): 22-24.
LU Dao-rong, LIN Jian-xin. The effect of bismuth on the cathodic copper deposition process of copper electrorefining[J]. Journal of Hefei University of Technology, 1996, 19(S1): 22-24.
[15] Girgis M, Ghali E. The electrorefining and winning of copper[M]. New York: A Publication of the Metallurgical Society, 1987: 173-193.
收稿日期:2008-07-21;修回日期:2008-10-11
基金项目:广东省创新基金资助项目(200501045)
通信作者:郑雅杰(1959-),男,湖南常德人,博士,教授,博士生导师,从事湿法冶金、水污染控制、资源综合利用研究;电话:0731-8836285;E-mail: zzyyjj01@yahoo.com.cn