镁盐沉淀法从钨酸盐溶液除磷的热力学研究
来源期刊:中国有色金属学报(英文版)2013年第11期
论文作者:何贵香 何利华 赵中伟 陈星宇 高利利 刘旭恒
文章页码:3440 - 3447
Key words:tungstate; phosphorus removal; thermodynamics; magnesium ammonium phosphate; chemical precipitation
摘 要:通过计算,绘制了25 °C时Mg2+- - -H2O体系的热力学平衡图,并对钨酸盐溶液除磷过程进行系统的热力学研究。结果表明:当采用磷酸镁盐法除磷时,溶液中游离总镁浓度从0.01 mol/L增加到1.0 mol/L,对应的最佳理论除磷pH值从9.8降到8.8,而溶液中残留的总磷含量基本维持在4.0×10-6 mol/L;随着溶液pH值的升高,体系中稳定存在的沉淀组分依次为MgHPO4、Mg3(PO4)2和Mg3(PO4)2+Mg(OH)2。当采用磷酸铵镁法除磷时,增大溶液游离总氨浓度有利于除磷,而增大游离总镁量对除磷深度基本无影响。计算所得除磷的最佳pH值为9~10;当游离总氨浓度为5.0 mol/L时,溶液中残留的总磷为1.4×10-7 mol/L。以自制的钨酸铵溶液(WO3 50 g/L,P 13 g/L)为原料,采用磷酸铵镁盐法除磷以对理论分析进行验证。结果表明:当氯化镁的加入量为理论量的1.1时,除磷的最佳pH值为9.5,与热力学分析结果一致。
Abstract: The thermodynamic equilibrium diagrams of Mg2+- - -H2O system at 298 K were established based on the thermodynamic calculation. From the diagram, the thermodynamic conditions for removing phosphorus from the tungstate solution by magnesium salt precipitation were obtained. The results show that when the concentration of total magnesium increases from 0.01 mol/L to 1.0 mol/L, the optimal pH for the phosphorus removal by magnesium phosphate decreases from 9.8 to 8.8. The residual concentration of total phosphorus almost keeps the level of 4.0×10-6 mol/L in the system. MgHPO4, Mg3(PO4)2 and the mixture of Mg3(PO4)2 and Mg(OH)2 are stabilized in these system, respectively. However, increasing the total concentration of magnesium has little effect on phosphorus removal by magnesium ammonium phosphate, while it is helpful for phosphorus removal by increasing the total ammonia concentration. The calculated results demonstrate that the residual concentration of total phosphorus can decrease to 5.0×10-7 mol/L as the total concentration of ammonia reaches 5.0 mol/L and the optimal pH value is 9-10. Finally, verification experiments were conducted with home-made ammonium tungstate solution containing 50 g/L WO3 and 13 g/L P. The results show that when the dosage of MgCl2 is 1.1 times of the theoretical amount, the optimum pH for removing phosphorus is 9.5, which matches with the results of the theoretical calculation exactly.
Trans. Nonferrous Met. Soc. China 23(2013) 3440-3447
Gui-xiang HE1, Li-hua HE1, Zhong-wei ZHAO1,2, Xing-yu CHEN1, Li-li GAO1, Xu-heng LIU1
1. School of Metallurgy and Environment, Central South University, Changsha 410083, China;
2. National Engineering Laboratory for High-Efficiency Recovery of Refractory Nonferrous Metal Resources, Central South University, Changsha 410083, China
Received 31 December 2012; accepted 15 March 2013
Abstract: The thermodynamic equilibrium diagrams of Mg2+- -
-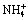 -H2O system at 298 K were established based on the thermodynamic calculation. From the diagram, the thermodynamic conditions for removing phosphorus from the tungstate solution by magnesium salt precipitation were obtained. The results show that when the concentration of total magnesium increases from 0.01 mol/L to 1.0 mol/L, the optimal pH for the phosphorus removal by magnesium phosphate decreases from 9.8 to 8.8. The residual concentration of total phosphorus almost keeps the level of 4.0×10-6 mol/L in the system. MgHPO4, Mg3(PO4)2 and the mixture of Mg3(PO4)2 and Mg(OH)2 are stabilized in these system, respectively. However, increasing the total concentration of magnesium has little effect on phosphorus removal by magnesium ammonium phosphate, while it is helpful for phosphorus removal by increasing the total ammonia concentration. The calculated results demonstrate that the residual concentration of total phosphorus can decrease to 5.0×10-7 mol/L as the total concentration of ammonia reaches 5.0 mol/L and the optimal pH value is 9-10. Finally, verification experiments were conducted with home-made ammonium tungstate solution containing 50 g/L WO3 and 13 g/L P. The results show that when the dosage of MgCl2 is 1.1 times of the theoretical amount, the optimum pH for removing phosphorus is 9.5, which matches with the results of the theoretical calculation exactly.
-H2O system at 298 K were established based on the thermodynamic calculation. From the diagram, the thermodynamic conditions for removing phosphorus from the tungstate solution by magnesium salt precipitation were obtained. The results show that when the concentration of total magnesium increases from 0.01 mol/L to 1.0 mol/L, the optimal pH for the phosphorus removal by magnesium phosphate decreases from 9.8 to 8.8. The residual concentration of total phosphorus almost keeps the level of 4.0×10-6 mol/L in the system. MgHPO4, Mg3(PO4)2 and the mixture of Mg3(PO4)2 and Mg(OH)2 are stabilized in these system, respectively. However, increasing the total concentration of magnesium has little effect on phosphorus removal by magnesium ammonium phosphate, while it is helpful for phosphorus removal by increasing the total ammonia concentration. The calculated results demonstrate that the residual concentration of total phosphorus can decrease to 5.0×10-7 mol/L as the total concentration of ammonia reaches 5.0 mol/L and the optimal pH value is 9-10. Finally, verification experiments were conducted with home-made ammonium tungstate solution containing 50 g/L WO3 and 13 g/L P. The results show that when the dosage of MgCl2 is 1.1 times of the theoretical amount, the optimum pH for removing phosphorus is 9.5, which matches with the results of the theoretical calculation exactly.
Key words: tungstate; phosphorus removal; thermodynamics; magnesium ammonium phosphate; chemical precipitation
1 Introduction
Tungsten is an important metal in many industrial and military applications due to its properties of high melting point, high hardness, large density and high strength at elevated temperature [1-4]. Its high-purity products are widely used in steel production, electronic engineering, electro-vacuum technologies and so on. In order to obtain high-purity tungsten products, the level of impurities in tungsten products must be reduced to only a few ppm. Otherwise, the properties of these products will be impaired. Phosphorus is one of the main impurity elements in tungsten metallurgical process and should be strictly limited in the final products. For example, based on the Chinese National Standard GB 10116—2007 APT-0, the content of phosphorus impurity requires less than 0.0007% (mass fraction). As is well known, ammonium paratungstate (APT), as the raw material for deep-processing tungsten products, is one of the most important intermediate products in the tungsten metallurgy. However, with the development of tungsten industry and the increasing consumption of tungsten concentrates resources, more and more low-grade, complex ores and secondary tungsten resources are used to produce APT, which leads to more impurity elements to dissolve into the leaching solution and then enter into the APT. Therefore, in order to obtain high quality APT, impurities removal will become more difficult.
So far, much research work has been conducted to remove phosphorus from aqueous solution and many methods have been invented. Biological methods and chemical methods are the major techniques [5,6]. Chemical methods for phosphorus removal have been widely used in industrial production for many years, including chemical precipitation, adsorption, solvent extraction [7], ion exchange [8-10], etc. Magnesium salt, calcium salt [11], iron and ferric salt [12], aluminum salt [13] and manganese salt are often used as precipitant to remove phosphorus [5,14,15]. In the field of tungsten metallurgy, magnesium salt, including magnesium chloride and magnesium sulphate, is the effective phosphorus removal reagent and has been used for many years [14]. Magnesium salt methods are carried out for removing phosphorus by the precipitation of magnesium ammonium phosphate and magnesium phosphate. Although magnesium salt method has been widely applied in tungsten industry, the theoretical research of the phosphorus removal from the tungstate solution is insufficient and needs further research. At home and abroad, there are much thermodynamic analyses on precipitation-dissolution equilibrium of the magnesium ammonium phosphate-water system [16-18]. JIANG et al [19] made the thermodynamic analysis on the 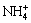 -Mg2+-
-Mg2+-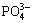 -H+-H2O system. In this system, the equilibrium concentration of ammonium is set very low. However, the concentration of ammonia is very high in the tungstate solution. It is obvious that JIANG’s thermodynamic analysis cannot be applied to the phosphorus removal from the tungstate solution.
-H+-H2O system. In this system, the equilibrium concentration of ammonium is set very low. However, the concentration of ammonia is very high in the tungstate solution. It is obvious that JIANG’s thermodynamic analysis cannot be applied to the phosphorus removal from the tungstate solution.
Based on above analysis, in order to get more information of the precipitation characteristics and obtain the predominance-area and the optimal conditions for phosphorus removal from tungstate solution by magnesium salt theoretically, it needs to carry out the thermodynamic research on the Mg2+-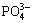 -
-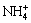 - H2O system based on the phosphorus removal from tungstate solution with magnesium salt method.
- H2O system based on the phosphorus removal from tungstate solution with magnesium salt method.
2 Data treatment and calculation model
In the Mg2+-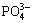 -
-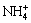 -H2O system, 16 species, H+, OH-, NH3,
-H2O system, 16 species, H+, OH-, NH3, 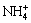 ,
, 
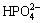 ,
, 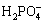 , H3PO4, Mg2+, MgOH+,
, H3PO4, Mg2+, MgOH+, 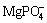 , MgHPO4,
, MgHPO4, 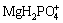 ,
, 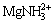 ,
,  ,
, 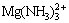 , are assumed to exist in the solution and 4 insoluble compounds are assumed to exist: MgHPO4, MgNH4PO4, Mg3(PO4)2, Mg(OH)2. Based on the literature data [16], the possible reaction equations and equilibrium constants are listed in Table 1.
, are assumed to exist in the solution and 4 insoluble compounds are assumed to exist: MgHPO4, MgNH4PO4, Mg3(PO4)2, Mg(OH)2. Based on the literature data [16], the possible reaction equations and equilibrium constants are listed in Table 1.
According to the reaction equations from Table 1, the equilibrium concentration equations of soluble components are as follows:
[H+][OH-]=10-14 (1)
[H+][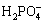 ]=10-2.04[H3PO4] (2)
]=10-2.04[H3PO4] (2)
[H+][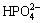 ]=10-7.2[
]=10-7.2[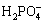 ] (3)
] (3)
[H+][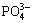 ]=10-12.36[
]=10-12.36[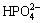 ] (4)
] (4)
[H+][NH3] =10-9.24[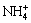 ] (5)
] (5)
[MgOH+]=102.57[Mg2+][OH-] (6)
[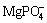 ]=104.8[
]=104.8[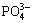 ][Mg2+] (7)
][Mg2+] (7)
[MgHPO4] =102.91[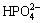 ][Mg2+] (8)
][Mg2+] (8)
[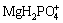 ]=100.45[Mg2+][
]=100.45[Mg2+][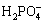 ] (9)
] (9)
[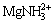 ]=100.24[NH3][Mg2+] (10)
]=100.24[NH3][Mg2+] (10)
[ ]=100.2[NH3]2[Mg2+] (11)
]=100.2[NH3]2[Mg2+] (11)
[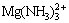 ]=10-0.3[NH3]3[Mg2+] (12)
]=10-0.3[NH3]3[Mg2+] (12)
[Mg2+][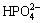 ]=10-5.5 (13)
]=10-5.5 (13)
[Mg2+][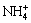 ][
][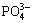 ]=10-12.6 (14)
]=10-12.6 (14)
[Mg2+]3[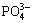 ]2=10-24.38 (15)
]2=10-24.38 (15)
[Mg2+][OH-]2=10-10.74 (16)
where [M] is the equilibrium concentration for each species.
Table 1 Equilibrium reactions and constants for Mg2+- -
- 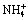 -H2O system at 298 K
-H2O system at 298 K

Besides, according to the law of mass conservation, mass balance equations are given as follows:
[Mg]T= [Mg2+]+[MgOH+]+[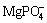 ]+[MgHPO4]+[
]+[MgHPO4]+[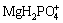 ]+[
]+[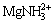 ]+[
]+[ ]+[
]+[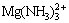 ] (17)
] (17)
[P]T = [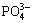 ]+[
]+[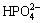 ]+[
]+[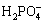 ]+[H3PO4]+[
]+[H3PO4]+[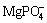 ]+[MgHPO4]+[
]+[MgHPO4]+[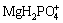 ] (18)
] (18)
[N]T= [NH3]+[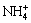 ]+[
]+[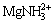 ]+[
]+[ ]+[
]+[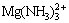 ] (19)
] (19)
where [Mg]T, [P]T and [N]T are designated as the total concentration of magnesium, phosphorus, ammonium under equilibrium conditions, respectively.
The calculation process based on the Newton- Raphson iteration method is carried out by using Microsoft EXCEL. Considering the lack of the activity coefficient data of the soluble component, the activity was replaced by concentration in practical calculation.
Based on the practical production experience of phosphorus removal from tungstate solution, the actual variables are the dosage of magnesium salt, the concentration of ammonium salt and pH of the solution. Therefore, the lg [M]-pH diagrams of phosphorus- containing species and lg [P]T-pH diagrams of the total concentration of P under conditions of fixed total concentration of Mg and ammonium contents were drawn.
3 Results
In practice, the phosphorus removal methods from tungstate solution mainly consist of magnesium phosphate precipitation method and magnesium ammonium phosphate precipitation method. The thermodynamic analysis of the two methods will be shown as follows.
3.1 Thermodynamics of phosphorus removal by magnesium phosphate precipitation method
The lg [P]T-pH diagram of Mg2+-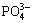 -H2O system for different [Mg]T concentration and the lg[M]-pH diagram are shown in Figs. 1 and 2 respectively. Figure 1 shows that the curve of the lg[P]T vs pH is approximately like the shape of the letter “V”. With increasing pH value, the stable precipitates are MgHPO4, Mg3(PO4)2 and the mixture of Mg3(PO4)2 and Mg(OH)2. Improving the [Mg]T concentration, the predominant-area of the Mg3(PO4)2 and Mg(OH)2 will expand to the direction of low pH (see the dotted lines ①-⑤ in Fig. 1). When the concentration of [Mg]T increases from 0.01 mol/L to 1.0 mol/L, the theoretical optimal pH for phosphorus removal by magnesium phosphate precipitation method decreases from 9.8 to 8.8 and the residual concentration of phosphorus basically maintains at the level of 4.0×10-6 mol/L. So, increasing the total concentration of Mg has little influence on phosphorus removal.
-H2O system for different [Mg]T concentration and the lg[M]-pH diagram are shown in Figs. 1 and 2 respectively. Figure 1 shows that the curve of the lg[P]T vs pH is approximately like the shape of the letter “V”. With increasing pH value, the stable precipitates are MgHPO4, Mg3(PO4)2 and the mixture of Mg3(PO4)2 and Mg(OH)2. Improving the [Mg]T concentration, the predominant-area of the Mg3(PO4)2 and Mg(OH)2 will expand to the direction of low pH (see the dotted lines ①-⑤ in Fig. 1). When the concentration of [Mg]T increases from 0.01 mol/L to 1.0 mol/L, the theoretical optimal pH for phosphorus removal by magnesium phosphate precipitation method decreases from 9.8 to 8.8 and the residual concentration of phosphorus basically maintains at the level of 4.0×10-6 mol/L. So, increasing the total concentration of Mg has little influence on phosphorus removal.

Fig. 1 Effect of total concentration of magnesium on phosphorus removal
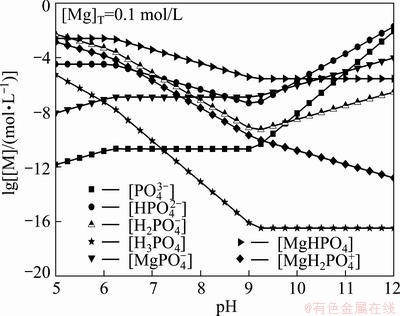
Fig. 2 Concentration of each phosphorus-containing species ([Mg]T=0.1 mol/L)
It is noteworthy that, with the increase of the [Mg]T concentration, the right part of the “V” letter-like curves almost overlaps, while the left part is some separated curves. It means that adding more magnesium salt has no effect on the deep removal of phosphorus when pH value is above 10. But as the pH value is below 10, enhancing the Mg content will slightly reduce the residual [P]T concentration in the solution. When the pH value exceeds 10, the Mg(OH)2 precipitation is generated. Mg2+ ion concentration depends on the dissolution equilibrium of the Mg(OH)2 precipitation. Meanwhile, the 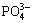 ion concentration is controlled by the dissolution equilibrium of the Mg3(PO4)2 precipitation. In this case, when pH value is constant, Mg2+ ion concentration will be invariable, and
ion concentration is controlled by the dissolution equilibrium of the Mg3(PO4)2 precipitation. In this case, when pH value is constant, Mg2+ ion concentration will be invariable, and 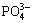 ion concentration will be an invariant after Mg3(PO4)2 precipitate generated. The concentration of all the phosphorus-containing species is invariable, namely, the total concentration of P is a constant value. So these curves are in coincidence. However, as for the left part it is different. When the pH value ranges from 5 to 9.5, the phosphorus exists mainly in the form of MgHPO4, and the magnesium exists mainly as Mg2+. When the concentration of [Mg]T increases to n times the original concentration, the concentration of Mg2+ will also amplify roughly to n times. In contrast, the concentration of
ion concentration will be an invariant after Mg3(PO4)2 precipitate generated. The concentration of all the phosphorus-containing species is invariable, namely, the total concentration of P is a constant value. So these curves are in coincidence. However, as for the left part it is different. When the pH value ranges from 5 to 9.5, the phosphorus exists mainly in the form of MgHPO4, and the magnesium exists mainly as Mg2+. When the concentration of [Mg]T increases to n times the original concentration, the concentration of Mg2+ will also amplify roughly to n times. In contrast, the concentration of 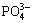 will decrease to n-3/2 times, according to dissolution equilibrium Eq. (15) of Mg3(PO4)2 ([Mg2+]3×[
will decrease to n-3/2 times, according to dissolution equilibrium Eq. (15) of Mg3(PO4)2 ([Mg2+]3×[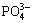 ]2 =10-24.38). At this point, it is easy to know that the concentration of HPO42- will also lessen to n -3/2 times. Furthermore, according to reaction Eq. (8), [MgHPO4(aq)]/([Mg2+]×[
]2 =10-24.38). At this point, it is easy to know that the concentration of HPO42- will also lessen to n -3/2 times. Furthermore, according to reaction Eq. (8), [MgHPO4(aq)]/([Mg2+]×[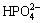 ])=102.91, the value of [MgHPO4(aq)] will decline to n-1/2 times when the value of [Mg2+]×[
])=102.91, the value of [MgHPO4(aq)] will decline to n-1/2 times when the value of [Mg2+]×[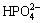 ] decreases to n×n-3/2=n-1/2 times. Therefore, when [Mg]T increases to n times, [P]T decreases to n-1/2 times. The curve moves downward.
] decreases to n×n-3/2=n-1/2 times. Therefore, when [Mg]T increases to n times, [P]T decreases to n-1/2 times. The curve moves downward.
3.2 Thermodynamics of magnesium ammonium phosphate precipitation method
As mentioned above, the phosphorus can also be removed from ammonium tungstate solution as magnesium ammonium phosphate when the magnesium salt is added into the solution. The thermodynamic analysis on the removal of phosphorus was conducted from two aspects as follows.
3.2.1 Effect of total magnesium concentration on phosphorus removal
Figure 3 shows the lg[P]T-pH diagram of Mg2+-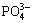 -
-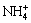 -H2O system for different total Mg contents when [N]T is 1.0 mol/L. Figure 4 shows the lg[M]-pH diagram of the phosphorus-containing species at 0.5 mol/L [Mg]T. Figure 3 shows that with the increase of pH value, the stable precipitates are, in order, MgHPO4, MgNH4PO4 and the mixture of MgNH4PO4 and Mg(OH)2. And with increasing the total concentration of Mg, the predominant-area of the Mg(OH)2 will expand to the direction of lower pH as the dotted lines ①-④ as shown in Fig. 3. Furthermore, the optimal theoretical pH value for dephosphorization is between 9 and 10, corresponding with the [P]T concentration of 5.0×10-7 mol/L (1.6×10-5 g/L). Compared with the magnesium phosphate precipitation method, the method with magnesium ammonium phosphate precipitation is more efficient for deep dephosphorization. Hence, in the optimum precipitation pH range, more Mg(OH)2 precipitate can only be obtained by increasing the dosage of the magnesium salt, which has little effect on the total concentration of P.
-H2O system for different total Mg contents when [N]T is 1.0 mol/L. Figure 4 shows the lg[M]-pH diagram of the phosphorus-containing species at 0.5 mol/L [Mg]T. Figure 3 shows that with the increase of pH value, the stable precipitates are, in order, MgHPO4, MgNH4PO4 and the mixture of MgNH4PO4 and Mg(OH)2. And with increasing the total concentration of Mg, the predominant-area of the Mg(OH)2 will expand to the direction of lower pH as the dotted lines ①-④ as shown in Fig. 3. Furthermore, the optimal theoretical pH value for dephosphorization is between 9 and 10, corresponding with the [P]T concentration of 5.0×10-7 mol/L (1.6×10-5 g/L). Compared with the magnesium phosphate precipitation method, the method with magnesium ammonium phosphate precipitation is more efficient for deep dephosphorization. Hence, in the optimum precipitation pH range, more Mg(OH)2 precipitate can only be obtained by increasing the dosage of the magnesium salt, which has little effect on the total concentration of P.
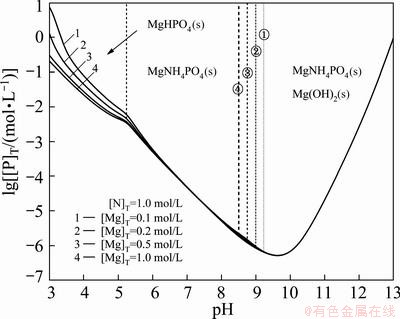
Fig. 3 Effect of total concentration of magnesium on phosphorus removal

Fig. 4 Concentration of each phosphorus-containing species ([N]T=1.0 mol/L, [Mg]T=0.5 mol/L)
Moreover, it is worth noting that the curves of lg[P]T-pH for different [Mg]T concentrations almost appear to overlap in the pH range of 6-13 due to the relationship between the magnesium ion concentration and pH value. When pH value exceeds 9, according to Eqs. (14) and (16), Mg2+ ion concentration is a constant and the equilibrium concentration of 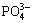 maintains unchanged. Thus, the concentration of all the phosphorus- containing species is invariable, in other words, the total concentration of P is a constant value. But in the pH range of 5.5-9, in which the predominant area is MgNH4PO4, the phosphorus exists mainly in the form of MgHPO4 and magnesium exists mainly in the form of Mg2+. When the [N]T concentration is a constant value and the concentration of [Mg]T increases to m times, the concentration of Mg2+ will increase roughly to m times. According to Eq. (14), the equilibrium concentration of
maintains unchanged. Thus, the concentration of all the phosphorus- containing species is invariable, in other words, the total concentration of P is a constant value. But in the pH range of 5.5-9, in which the predominant area is MgNH4PO4, the phosphorus exists mainly in the form of MgHPO4 and magnesium exists mainly in the form of Mg2+. When the [N]T concentration is a constant value and the concentration of [Mg]T increases to m times, the concentration of Mg2+ will increase roughly to m times. According to Eq. (14), the equilibrium concentration of 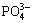 will decrease to 1/m times. The corresponding concentration of
will decrease to 1/m times. The corresponding concentration of 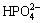 will also drop to 1/m times and the product of [Mg2+]×[
will also drop to 1/m times and the product of [Mg2+]×[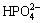 ] is a constant. Based on the constant of [MgHPO4]/([Mg2+]×[
] is a constant. Based on the constant of [MgHPO4]/([Mg2+]×[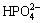 ])= 102.91, the concentration of MgHPO4 keeps the same from beginning to end. So, the [P]T concentration maintains unchanged. However, when the pH ranges from 3 to 5, the effect of dephosphorization will be better (shown as the curve 1→4 in Fig. 3) with the total concentration of Mg increasing. The reason is that the phosphorus exists mainly in the form of
])= 102.91, the concentration of MgHPO4 keeps the same from beginning to end. So, the [P]T concentration maintains unchanged. However, when the pH ranges from 3 to 5, the effect of dephosphorization will be better (shown as the curve 1→4 in Fig. 3) with the total concentration of Mg increasing. The reason is that the phosphorus exists mainly in the form of 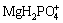 ,
, 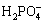 and MgHPO4 (shown in Fig. 4). The concentrations of
and MgHPO4 (shown in Fig. 4). The concentrations of 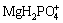 and MgHPO4 remain unchanged at a fixed pH value (shown in Fig. 5), while the concentration of
and MgHPO4 remain unchanged at a fixed pH value (shown in Fig. 5), while the concentration of 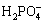 decreases when the [Mg]T concentration increases.
decreases when the [Mg]T concentration increases.
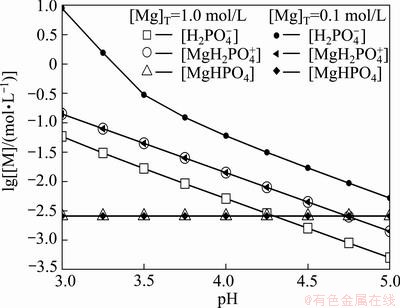
Fig. 5 Concentration of each phosphorus-containing species ([N]T=1.0 mol/L)
3.2.2 Effect of total concentration of ammonia on phosphorus removal
Figure 6 shows the lg[P]T-pH diagram of Mg2+- -
- -H2O system for different [N]T with 0.1 mol/L [Mg]T. It indicates that the [P]T concentration decreases from 4.0×10-6 mol/L to 1.4×10-7 mol/L in the pH range of 6-13 as the [N]T concentration increases from 0.1 mol/L to 5.0 mol/L. The optimal theoretical pH is in the range of 9-10. The phosphorus will be removed as Mg3(PO4)2 in low concentration of ammonia and MgNH4PO4 in high concentration of ammonia.
-H2O system for different [N]T with 0.1 mol/L [Mg]T. It indicates that the [P]T concentration decreases from 4.0×10-6 mol/L to 1.4×10-7 mol/L in the pH range of 6-13 as the [N]T concentration increases from 0.1 mol/L to 5.0 mol/L. The optimal theoretical pH is in the range of 9-10. The phosphorus will be removed as Mg3(PO4)2 in low concentration of ammonia and MgNH4PO4 in high concentration of ammonia.
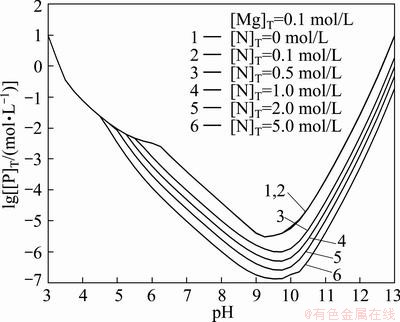
Fig. 6 Effect of total concentration of ammonia on phosphorus removal ([Mg]T=0.1 mol/L)
Figure 7 shows the lg[P]T-pH diagram of Mg2+-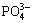 -
-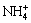 -H2O system for different [N]T concentration ([N]T=0.1, 0.15, 0.2, 5.0 mol/L) at 0.1 mol/L [Mg]T. Compared with Fig. 3, the precipitate generated by magnesium ammonium phosphate method is different along with the concentration. There are three typical forms in conclusion.
-H2O system for different [N]T concentration ([N]T=0.1, 0.15, 0.2, 5.0 mol/L) at 0.1 mol/L [Mg]T. Compared with Fig. 3, the precipitate generated by magnesium ammonium phosphate method is different along with the concentration. There are three typical forms in conclusion.
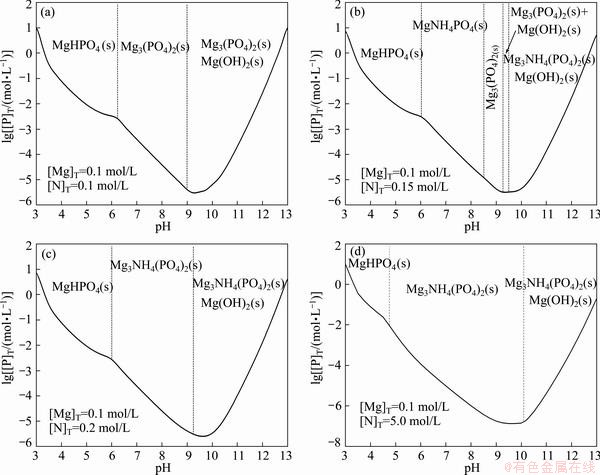
Fig. 7 Predominance-area diagrams of precipitation at different total ammonia concentration ([Mg]T=0.1 mol/L)
The predominant areas are MgHPO4, Mg3(PO4)2, and mixture of Mg3(PO4)2+Mg(OH)2, respectively, with the pH value increasing from 3 to 13 at 0.1 mol/L [N]T. The predominant areas are MgHPO4, MgNH4PO4, Mg3(PO4)2, mixture of Mg3(PO4)2+Mg(OH)2 and mixture of MgNH4PO4+Mg(OH)2 at 0.15 mol/L [N]T. The predominant areas are MgHPO4, MgNH4PO4 and mixture of MgNH4PO4+Mg(OH)2 when [N]T is over 0.2 mol/L. From the changes of the precipitate, it can be found that Mg3(PO4)2 transforms into more stable precipitate MgNH4PO4 thermodynamically and the predominant area of MgNH4PO4 tends to expand with the increase of the concentration of total ammonia. Based on the change tendency, the [N]T concentration must be over the critical concentration to form the MgNH4PO4(s) for phosphorus removal from tungstate solution by magnesium ammonium phosphate precipitation method.
4 Discussion
The above analysis indicates that the residual P concentration remains to be 4.0×10-6 mol/L after phosphorus removal from tungstate solution by magnesium phosphate precipitation method when the [Mg]T concentration increases from 0.01 mol/L to 1.0 mol/L. Increasing the dosage of magnesium salt has no effect on the deep removal of phosphorus. Similarly, when phosphorus is removed by magnesium ammonium phosphate precipitation method, increasing the dosage of magnesium salt has little effect in the optimum pH range of 9-10 (Fig. 3).
However, PENG et al [20] indicated that increasing the concentration of total Mg is beneficial for phosphorus removal. The production practices also seem to have proved that. Obviously, the result is contradicted with our theoretical research results. In recent research on the reason for the different analysis results, it is found that the phosphorus-containing species of MgHPO4 is an important component in the system containing Mg2+ and 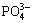 ions [19,21-23]. And the calculation results in this work indicate that the phosphorus-containing ion is mainly MgHPO4 in the pH range of 5-10 during the dephosphorization process. But in Ref. [20], the MgHPO4 is not taken into account. Ignoring this species will lead to large deviation. Actually, with a further analysis, we find that the deep removal of phosphorus is limited and is far from the thermodynamic equilibrium. Increasing the dosage of the magnesium salt just only accelerates the kinetics rate of the reaction and makes the reaction approach to the state of the thermodynamic equilibrium, but it has no effect on the deep removal of phosphorus.
ions [19,21-23]. And the calculation results in this work indicate that the phosphorus-containing ion is mainly MgHPO4 in the pH range of 5-10 during the dephosphorization process. But in Ref. [20], the MgHPO4 is not taken into account. Ignoring this species will lead to large deviation. Actually, with a further analysis, we find that the deep removal of phosphorus is limited and is far from the thermodynamic equilibrium. Increasing the dosage of the magnesium salt just only accelerates the kinetics rate of the reaction and makes the reaction approach to the state of the thermodynamic equilibrium, but it has no effect on the deep removal of phosphorus.
5 Verification experiment
According to the above theoretical analysis results, it is known that pH value plays an important role in the deep removal of phosphorus from tungstate solution. In order to verify the theoretical validity, verification experiments were done as follows.
First, 50 mL ammonium tungstate solution (WO3 50 g/L, P 13 g/L) home-made was added into a beaker, and the pH of the aqueous solution was adjusted with hydrochloric acid or aqueous ammonia to a certain value under magnetic stirring. Then, the prepared MgCl2 solution (MgCl2 190 g/L, the dosage of the magnesium chloride was 1.1 times stoichiometric amount) was dropped with pipette, stirring for about 1 h and then filtered.
The contents of P and WO3 were analyzed by inductively coupled plasma (ICP) method. The ratio of dephosphorization and the loss of WO3 were calculated according to the analysis results. The relationship between pH and the effect of the dephosphorization is shown in Table 2.
Table 2 Relationship between pH and removal rate of phosphorus
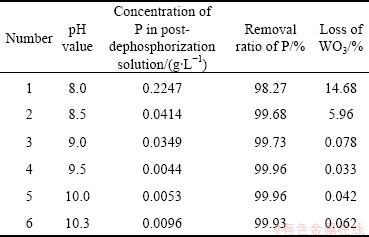
Table 2 shows that the pH value does have a large impact on the removal rate of phosphorus. The removal ratio increases first and then decreases with increasing the pH value. The optimal pH value for dephosphorization is 9.5. The residual concentrations of phosphorus and magnesium are 4.4 mg/L (1.4×10-4 mol/L) and 33 mg/L, respectively, and mass ratio of P to WO3 is 8.8×10-5 after dephosphorization at pH 9.5. The removal rate of phosphorus is 99.96%. The verification experimental results show that the optimal pH value of phosphorus removal from ammonium tungstate solution is basically consistent with the above theoretical analysis results, which means that our theoretical analyses are correct.
6 Conclusions
1) Based on the literature data, thermodynamic calculation was conducted and the thermodynamic equilibrium diagrams of Mg2+-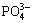 -
-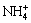 -H2O system at 298 K are drawn. The optimal technological conditions for phosphorus removal from tungstate solution are theoretically predicted.
-H2O system at 298 K are drawn. The optimal technological conditions for phosphorus removal from tungstate solution are theoretically predicted.
2) In the process of the phosphorus removal by magnesium phosphate precipitation method, when the concentration of total Mg increases from 0.01 mol/L to 1.0 mol/L, the residual total P concentration remains at 4.0×10-6 mol/L and the optimal pH value decreases from 9.8 to 8.8.
3) In the process of the phosphorus removal by magnesium ammonium phosphate precipitation method, the theoretical optimum pH range is 9-10 and the corresponding total P concentration is 1.4×10-7 mol/L when the total concentration of ammonia is 5.0 mol/L. So, the magnesium ammonium phosphate precipitation method is superior to the magnesium phosphate precipitation method in the deep removal of phosphorus.
4) Verification experimental results demonstrate that the optimum pH is 9.5. The removal rate of phosphorus is 99.96% when the dosage of magnesium chloride is 1.1 times theoretical amount. The experimental results are basically consistent with the above theoretical analysis results, which means our theoretical analyses are correct.
References
[1] WANG Xu, LIAO Chun-fa, YANG Wen-qiang, XIE Quan-wen. Characterization and electrochemical analysis of tungsten powder prepared by molten salt electrolysis in CaWO4-CaCl2-NaCl system [J]. The Chinese Journal of Nonferrous Metals, 2012, 22(5): 1482-1487. (in Chinese)
[2] LI Xiao-qiang, XIN Hong-wei, HU Ke, LI Yuan-yuan. Microstructure and properties of ultra-fine tungsten heavy alloys prepared by machanical alloying and electric current activated sintering [J]. Transactions of Nonferrous Metals Society of China, 2010, 20(3): 443-449.
[3] ZHU Y B, WANG Y, ZHANG X Y, QIN G W. W/NiFe phase interfacial characteristics of liquid-phase sintered W-Ni-Fe alloy [J]. International Journal of Refractory Metals and Hard Materials, 2007, 25(4): 275-279.
[4] TAN Jun, ZHOU Zhang-jian, ZHU Xiao-peng, GUO Shuang-quan, QU Dan-dan, LEI Ming-kai, GE Chang-chun. Evaluation of ultra-fine grained tungsten under transient high heat flux by high-intensity pulsed ion beam [J]. Transactions of Nonferrous Metals Society of China, 2012, 22: 1081-1085.
[5] de BASHAN L E, BASHAN Y. Recent advances in removing phosphorus from wastewater and its future use as fertilizer (1997-2003) [J]. Water Research, 2004, 38(19): 4222-4246.
[6] DUENAS J F, ALONSO J R, REY  F, FERRER A S. Characterisation of phosphorous forms in wastewater treatment plants [J]. Journal of Hazardous Materials, 2003, 97(1-3): 193-205.
F, FERRER A S. Characterisation of phosphorous forms in wastewater treatment plants [J]. Journal of Hazardous Materials, 2003, 97(1-3): 193-205.
[7] ZHAO You-cai, CHEN Jia-yong. Extraction of phosphorus, arsenic and/or silica from sodium tungstate and molybdate solutions with primary amine and tributyl phosphate as solvents. I: Synergistic extraction and separation of phosphorus, arsenic and/or silica from tungstate and molybdate solution [J]. Hydrometallurgy, 1996, 42(3): 313-324.
[8] AWUAL M R, JYO A. Assessing of phosphorus removal by polymeric anion exchangers [J]. Desalination, 2011, 281: 111-117.
[9] BLANEY L M, CINAR S, SENGUPTA A K. Hybrid anion exchanger for trace phosphate removal from water and wastewater [J]. Water Researcher, 2007, 41(7): 1603-1613.
[10] SENGUPTA S, PANDIT A. Selective removal of phosphorus from wastewater combined with its recovery as a solid-phase fertilizer [J].Water Research, 2011, 45(11): 3318-3330.
[11] SONG Y H, HAHN H H, HOFFMANN E. Effects of solution conditions on the precipitation of phosphate for recovery—A thermodynamic evaluation [J]. Chemosphere, 2002, 48(10): 1029-1034.
[12] FYTIANOS K, VOUDRIAS E, RAIKOS N. Modelling of phosphorous removal from aqueous and wastewater samples using ferric iron [J]. Environmental Pollution, 1998, 101(1): 123-130.
[13] HUANG S H, CHISWELL B. Phosphate removal from wastewater using spent alum sludge [J]. Water Science and Technology, 2000, 42(3-4): 295-300.
[14] PENG Shao-fang. Metallurgy of tungsten [M]. Beijing: Metallurgical Industry Press, 1981: 57-65. (in Chinese)
[15] ZHAO Zhong-wei, HUO Guang-sheng, LI Hong-gui, CHEN Ai-liang, LIU Jin. Removal methods of tungsten, vanadium, phosphorus, arsenic precipitation from molybdate solution: CN101492181A [P]. 2009-07-27. (in Chinese)
[16] MICHALOWSKI T, PIETRZYK A. A thermodynamic study of struvite+water system [J]. Talanta, 2006, 68(3): 594-601.
[17] HANHOUN M, MONTASTRUC L, AZZARO-PANTEL C, BISCANS B, FRECHE M, PIBOULEAU L. Temperature impact assessment on struvite solubility product: A thermodynamic modeling approach [J]. Chemical Engineering Journal, 2011, 167(1): 50-58.
[18] WANG Jian-sen, SONG Yong-hui, YUAN Peng, PENG Jian-feng, FAN Mao-hong. Modeling the crystallization of magnesium ammonium phosphate for phosphorus recovery [J]. Chemosphere, 2006, 65(7): 1182-1187.
[19] JIANG Ke, ZHOU Kang-gen, PENG Jia-le. Thermodynamic of solid-liquid equilibrium in  -Mg2+-
-Mg2+-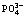 -H+-H2O system [J]. Journal of Central South University: Science and Technology, 2009, 40(5): 1178-1182. (in Chinese)
-H+-H2O system [J]. Journal of Central South University: Science and Technology, 2009, 40(5): 1178-1182. (in Chinese)
[20] PENG Shao-fang, ZHENG Chang-qiong, YIN Guang-fu. The thermodynamic analysis on eliminating phosphorus the process of the recovery of superalloy scrap [J]. Journal of Chengdu University of Science and Technology, 1985(2): 13-21. (in Chinese)
[21] SILVEIRA M L, MIYITTAH M K, O’CONNOR G A. Phosphorus release from a manure-impacted spodosol: Effects of a water treatment residual [J]. Journal of Environmental Quality, 2006, 35(2): 529-541.
[22] van RENSBURG P, MUSVOTO E V, WENTZEL M C, EKAMA G A. Modelling multiple mineral precipitation in anaerobic digester liquor [J]. Water Research, 2003, 37(13): 3087-3097.
[23]  L, KRALJ D. Kinetics of struvite to newberyite transformation in the precipitation system MgCl2-NH4H2PO4-NaOH-H2O [J]. Water Research, 2006, 40(18): 3447-3455.
L, KRALJ D. Kinetics of struvite to newberyite transformation in the precipitation system MgCl2-NH4H2PO4-NaOH-H2O [J]. Water Research, 2006, 40(18): 3447-3455.
何贵香1,何利华1,赵中伟1,2,陈星宇1,高利利1,刘旭恒1
1. 中南大学 冶金与环境学院,长沙 410083;
2. 中南大学 难冶有色金属资源高效利用国家工程实验室,长沙 410083
摘 要:通过计算,绘制了25 °C时Mg2+- -
-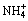 -H2O体系的热力学平衡图,并对钨酸盐溶液除磷过程进行系统的热力学研究。结果表明:当采用磷酸镁盐法除磷时,溶液中游离总镁浓度从0.01 mol/L增加到1.0 mol/L,对应的最佳理论除磷pH值从9.8降到8.8,而溶液中残留的总磷含量基本维持在4.0×10-6 mol/L;随着溶液pH值的升高,体系中稳定存在的沉淀组分依次为MgHPO4、Mg3(PO4)2和Mg3(PO4)2+Mg(OH)2。当采用磷酸铵镁法除磷时,增大溶液游离总氨浓度有利于除磷,而增大游离总镁量对除磷深度基本无影响。计算所得除磷的最佳pH值为9~10;当游离总氨浓度为5.0 mol/L时,溶液中残留的总磷为1.4×10-7 mol/L。以自制的钨酸铵溶液(WO3 50 g/L,P 13 g/L)为原料,采用磷酸铵镁盐法除磷以对理论分析进行验证。结果表明:当氯化镁的加入量为理论量的1.1时,除磷的最佳pH值为9.5,与热力学分析结果一致。
-H2O体系的热力学平衡图,并对钨酸盐溶液除磷过程进行系统的热力学研究。结果表明:当采用磷酸镁盐法除磷时,溶液中游离总镁浓度从0.01 mol/L增加到1.0 mol/L,对应的最佳理论除磷pH值从9.8降到8.8,而溶液中残留的总磷含量基本维持在4.0×10-6 mol/L;随着溶液pH值的升高,体系中稳定存在的沉淀组分依次为MgHPO4、Mg3(PO4)2和Mg3(PO4)2+Mg(OH)2。当采用磷酸铵镁法除磷时,增大溶液游离总氨浓度有利于除磷,而增大游离总镁量对除磷深度基本无影响。计算所得除磷的最佳pH值为9~10;当游离总氨浓度为5.0 mol/L时,溶液中残留的总磷为1.4×10-7 mol/L。以自制的钨酸铵溶液(WO3 50 g/L,P 13 g/L)为原料,采用磷酸铵镁盐法除磷以对理论分析进行验证。结果表明:当氯化镁的加入量为理论量的1.1时,除磷的最佳pH值为9.5,与热力学分析结果一致。
关键词:钨酸盐;除磷;热力学;磷酸铵镁;沉淀法
(Edited by Hua YANG)
Foundation item: Project (2012BAB10B04) supported by the National Key Technologies R&D Program of China
Corresponding author: Zhong-wei ZHAO; Tel: +86-731-88830476; Fax: +86-731-88830477; E-mail: zhaozw@csu.edu.cn
DOI: 10.1016/S1003-6326(13)62886-1

