Trans. Nonferrous Met. Soc. China 24(2014) s157-s161
Selective recovery of Sn from copper alloy dross and its heat-treatment for synthesis of SnO2
Jung-Il LEE1, Jong Bum PARK1, Tae Wan KIM1, Man-Sik KONG2, Jeong Ho RYU1
1. Department of Materials Science and Engineering, Korea National University of Transportation, Chungju, Chungbuk 380-702, Korea;
2. Functional Materials Research Team, Advanced Materials & Processing Center, Institute for Advanced Engineering, Yongin, Gyunggi 449-863, Korea
Received 18 June 2013; accepted 24 March 2014
Abstract: Preliminary study on concentration and separation of tin (Sn) from copper alloy dross by selective dissolution method was conducted. The tin in the copper alloy dross did not dissolve in an aqueous nitric acid solution which could allow separation of tin from the copper alloy dross. The tin as H2SnO3 (metastannic acid) phase was precipitated in the solution with centrifuging process and transformed to tin dioxide (SnO2) after drying process. The dried sample was heat-treated at low temperature and its phase characteristics, surface morphology and chemical composition were investigated.
Key words: recovery of Sn; copper alloy dross; selective dissolution; SnO2
1 Introduction
Tin dioxide (SnO2) is an important semiconducting material which has been widely used in an extensive range of applications, such as catalysts [1], gas sensors [2], heat mirrors [3], varistors [4], transparent electrodes for solar cells [5] and optoelectronic devices [6]. Tin dioxide-based gas sensors are very important n-type semiconductor sensors which can be utilized to detect various inflammable and harmful gases such as hydrogen (H2) and carbon dixoide (CO) [7]. The tin dioxide can be synthesized by various synthesis methods such as direct strike precipitation [8], two-step solid state synthesis [9], microemulsion [10], gel combustion technique [11] and hydrothermal synthesis [12].
Copper alloy dross resulting from pyrometallurgical copper processes is important by-products to be controlled in structure and chemical composition. Due to the significant volumes of dross compared with those of the target metal, it is mandatory to use the dross as a product. After matte smelting and standard dross cleaning in submerged arc furnaces, the alloy dross still contains copper and other valuable metals like nickel, cobalt or tin [13]. In addition, future regulations may restrict the heavy metal contents in ores to be decreased in the available deposits, much below the upper value of discarded dross. Therefore, sustainable dross management is necessary from the economic and environmental point of view.
Moreover, the processing of secondary materials for the recovery of valuable metals in an environmentally acceptable manner with low energy, capital and operating costs has been given due to attention in the metal extraction and recovery [14,15]. In order to recover copper, nickel or tin as value added product from the dross generated in a copper alloy smelter, a process comprising of concentration and separation steps was developed [16]. In this study, preliminary study on concentration and separation of tin from copper alloy dross with selective dissolution method using nitric acid is performed. The separated tin base precipitate is heat-treated to crystalline SnO2 and its phase characteristics, surface morphology and chemical composition are investigated.
2 Experimental
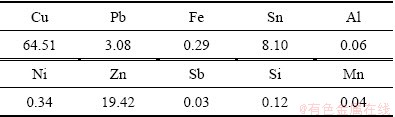
The copper alloy dross used in this study was served from Seowon Co., Ltd. The chemical composition was analyzed by X-ray fluorescence (XRF) as shown in Table 1. The nitric acid was in analytical grade without further purification. Dissolution experiment was carried out in a 2 L beaker and the concentration of nitric acid was fixed to 4 mol/L. To minimize the effect of the exothermic reaction of nitrous oxide fumes and hydrogen evolution, 10 g of the copper alloy dross was added to 500 mL aqueous nitric acid solution. Magnetic stirring was controlled at 200 r/min for uniform dissolving and the temperature of the solution was maintained at 80 °C for 3 h.
Table 1 Chemical composition of copper alloy dross (mass fraction, %)
After 3 h of dissolving time, the solution was centrifuged at 8000 r/min for 10 min in order to separate precipitate. The centrifuged precipitate was dried at 100 °C for 12 h. The phase analysis and thermal property of the precipitate was performed by X-ray diffraction (XRD) and thermogravimetric-differential thermal (TG-DT) analysis. The precipitate was calcined at 800 °C based on the TG-DT result and the heat-treated sample was investigated using XRD and scanning electron microscopy (SEM) for crystalline phase and surface morphology. The chemical composition of the heat-treated sample was analyzed using XRF.
3 Results and discussion
In general, the choice of a dissolving agent depends on various factors, which include the physical and chemical characteristics of the material to be dissolved, optimum selectivity, cost of the reagent and its ability to be regenerated [17]. The performance of the dissolving agent for the precipitate of tin in the copper alloy dross was investigated using aqueous nitric acid solution. Nitric acid is a strong oxidizing reagent which is able to corrode most of the metals in the copper alloy dross. Its selectivity, in view of dissolution properties for copper, nickel and tin of the dross, has considerable advantages over hydrochloric and sulfuric acid, which may cause problems due to the formation of undesirable precipitates [17]. In addition, the low cost of HNO3 and the possibility of its easy regeneration and re-use is attractive.
During dissolving the copper alloy dross in aqueous nitric acid solution, copper reacts to form copper nitrate according to the reaction:
3Cu+8HNO3=3Cu(NO3)2+4H2O+2NO (1)
Lead is dissolved by nitric acid to form soluble lead nitrate by the following reaction:
Pb+2HNO3=Pb(NO3)2+H2 (2)
Other important metals such as nickel and zinc also react with nitric acid. Nickel forms nickel(II) nitrate, and zinc is oxidized to zinc nitrate:
Ni+4HNO3=Ni(NO3)2+2H2O+2NO2 (3)
3Zn+8HNO3=3Zn(NO3)2+4H2O+2NO (4)
However, when tin is treated with nitric acid, a precipitate of hydrous stannic oxide (metastannic acid) is formed [18]:
Sn+4HNO3=H2SnO3↓+H2O+4NO2 (5)
Figure 1 shows the XRD pattern of the precipitated sample after washing and drying (i.e., uncalcined). Although the peaks are very broad, the XRD patterns confirm the presence of single phase SnO2 in rutile structure (tetragonal system, JSPDS 41-1445) for all peaks. This indicates that tin is precipitated in a metastannic acid phase during dissolving in the aqueous nitric acid, and subsequently crystallized to tin dioxide (SnO2) phase. During the subsequent drying process, H2SnO3 decomposed to produce H2O and SnO2. The evaporation of H2O can create nanopores in the SnO2 particles [19] and synthetic route for tin dioxide can be expressed as follows based on the XRD result:
H2SnO3 SnO2+H2O (6)
SnO2+H2O (6)
TG-DT analysis was performed to determine the thermal behavior of the precipitated phase and hence to choose an optimum calcination temperature. Figure 2 depicts TG and DT curves, for the precipitated sample. Total mass loss occurs in two stages as shown in TG curve. Firstly, mass loss is observed lower than 250 °C due to physically adsorbed water. Secondly, chemically adsorbed water is removed from the sample from 250 to 600 °C and this resulted in the endothermic peaks in DT curve. The result of TG-DT study indicates that when the precipitated sample is heated to 600 °C, all the reactions in the sample are completed. That is, further heating above 600 °C of the precipitated sample does not cause any thermal and/or weight changes. These TG-TD results are also in agreement with the previous studies using conventional synthetic routes [20].

Fig. 1 Morphology of dried precipitate after centrifugation (inset) and its XRD pattern

Fig. 2 TG-DTA curves for dried precipitate
Based on the TG-DT analysis, the precipitate was calcined at 700 °C for 1 h, and the XRD pattern for the heat-treated sample is shown in Fig. 3. The heat-treated sample exhibits single SnO2 phase with high crystallinity compared to the precipitate before heat-treatment. The crystallite sizes of the precipitate and heat-treated SnO2 samples were calculated by means of XRD line broadening measurement according to Scherrer equation [20]:
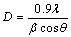 (7)
(7)
where D is the mean crystallite size; λ is the wavelength, i.e., 1.5418  for Cu Kα; β is the full width half maximum of SnO2 (110) line after instrumental broadening effects are eliminated; 2θ is the diffraction angle for the (110) line (in radian). The calculated crystallite size increases from 10.3 to 35.2 nm after heat-treatment.
for Cu Kα; β is the full width half maximum of SnO2 (110) line after instrumental broadening effects are eliminated; 2θ is the diffraction angle for the (110) line (in radian). The calculated crystallite size increases from 10.3 to 35.2 nm after heat-treatment.
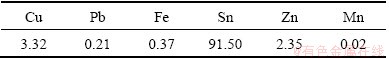
Table 2 shows the chemical composition of the heat-treated SnO2 sample measured by XRF analysis. As shown in Table 2, the heat-treated SnO2 sample is composed of 91.50% Sn, 3.32% Cu, 2.35% Zn, 0.21% Pb, 0.37% Fe and 0.02% Mn, which indicates that the selective dissolution method can be a simple and efficient separation method for tin metal from copper alloy dross.
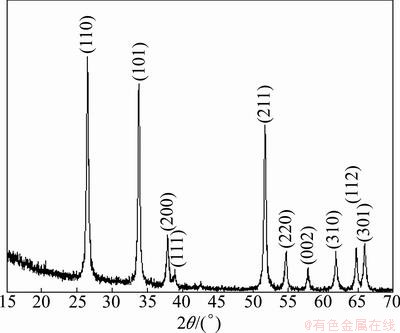
Fig. 3 XRD pattern of heat-treated precipitate at 700 °C for 1 h
Table 2 Chemical composition of heat-treated SnO2 sample measured by XRF (mass fraction, %)
The SEM micrograph and EDX spectrum of the heat-treated SnO2 sample at 700 °C is shown in Fig. 4. It shows an aggregate of very fine particles that cannot be virtually distinguished or measured using a SEM. The chemical composition measured by EDX is almost Sn and very small quantity of Cu, Si and Al, which is consistent with the XRF result.
4 Conclusions
The recovery and separation of tin (Sn) from copper alloy dross was successfully achieved by a selective dissolution method in aqueous nitric acid. The tin in the copper alloy dross didn’t dissolve in the aqueous nitric acid solution which could allow the concentration and separation of the tin from the copper alloy dross. The solid phase H2SnO3 was precipitated in the solution and transformed to tin dioxide (SnO2) after drying process. Highly crystallized SnO2 powder was successfully obtained by a low-temperature heat treatment, which indicates that the selective dissolution method can be a simple and efficient separation for valuable metal resource from copper alloy dross.
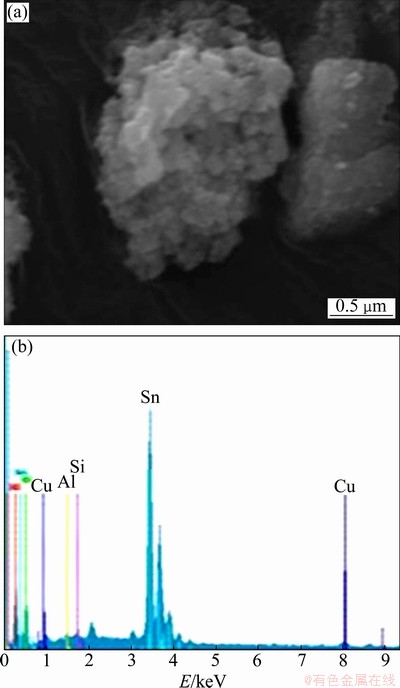
Fig. 4 SEM micrograph (a) and EDX spectrum of heat-treated SnO2 sample
Acknowledgement
This work was supported by the “Energy Efficiency & Resources” of the Korea Institute of Energy Technology Evaluation and Planning (KETEP) grant funded by the Korea government Ministry of Knowledge Economy (No. 20125010100030-11-2-400).
References
[1] CHOU L, CAI Y, ZHANG B, NIU J, JI S, LI S. Influence of SnO2-doped W-Mn/SiO2 for oxidative conversion of methane to high hydrocarbons at elevated pressure [J]. Applied Catalysis A, 2003, 238: 185-191.
[2] SHIMIZU Y, EGASHIRA M. Basic aspects and challenges of semiconductor gas sensors [J]. MRS Bulletin, 1999, 24(6): 18-24.
[3] LAMPERT C M. Heat mirror coating for energy conserving windows [J]. Solar Energy Materials, 1981, 6: 1-41.
[4] WANG J F, WANG Y J, SU W B, CHEN H C, WANG W X. Novel (Zn,Nb)-doped SnO2 varistors [J]. Materials Science and Engineering B, 2002, 96: 8-13.
[5] MOUSTAFID T E, CACHET H, TRIBOLLET B, FESTY D. Modified transparent SnO2 electrodes as efficient and stable cathodes for oxygen reduction [J]. Electrochimica Acta, 2002, 47(8): 1209-1215.
[6] CHANG S S, JO M S. Luminescence properties of Eu-doped SnO2 [J]. Ceramics International, 2007, 33: 511-514.
[7] WIERZCHOWSKI P T, ZATORSKI L W. Kinetics of catalytic oxidation of carbon monoxide and methane combustion over alumina supported Ga2O3, SnO2 or V2O5 [J]. Applied Catalysis B, 2003, 44(1): 53-65.
[8] SONG K C, KANG Y. Preparation of high surface area tin oxide powder by a homogeneous precipitation method [J]. Materials Letters, 2000, 42: 283-289.
[9] LI F, CHEN L, CHEN Z, XU J, ZHU J, XIN X. Two-step solid state synthesis of tin oxide and its gas-sensing property [J]. Materials Chemistry and Physics, 2002, 73: 335-338.
[10] SONG K C, KIM J H. Preparation of nanosize tin oxide particle from water-in-oil microemulsions [J]. Journal of Colloid and Interface Science, 1999, 212: 193-196.
[11] BHAGWAT M, SHAH P, RAMASWAMY V. Synthesis of nanocrystalline SnO2 powder by amorphous citrate route [J]. Materials Letters, 2003, 57: 1604-1611.
[12] BAIK N S, SAKAI G, MIURA N, YAMAZOE N. Preparation of stabilized nanosized tin oxide particles by hydrothermal treatment [J]. Journal of the American Ceramic Society, 2000, 83: 2983-2987.
[13] ARBITER N, FLETCHER A W. Copper hydrometallurgy-evolution and milestones [J]. Minerals Engineering, 1994, 46(2): 118-123.
[14] SUGIYAMA S, MERA T, YANAGIMOTO J. Recycling of minute metal scraps by semisolid processing: Manufacturing of design materials [J]. Transactions of Nonferrous Metals Society of China, 2010, 20: 1567-1571.
[15] ZHENG Ya-jie, PENG Ying-lin, KE L, CHEN Wen-mi. Separation and recovery of Cu and As from copper electrolyte through electrowinning and SO2 reduction [J]. Transactions of Nonferrous Metals Society of China, 2013, 23: 2166-2173.
[16] FERRAZ A I, TAVAVARES T, TEIXEIRA J A. Cr(III) removal and recovery from saccharomyces cerevisiae [J]. Chemical Engineering Journal, 2004, 105: 11-20.
[17] MECUCCI A, SCOTT K. Leaching and electrochemical recovery of copper, lead and tin from scrap printed circuit boards [J]. Journal of Chemical Technology and Biotechnology, 2002, 77: 449-457.
[18] AL-SUHYBANI A A. Effect of some inorganic anions on corrosion of tin in nitric acid [J]. British Corrosion Journal, 1989, 24(3): 204-210.
[19] WANG H, SUN F, ZHANG Y, LI L, CHEN H, WU Q, YU J C. Photochemical growth of nanoporous SnO2 at the air-water interface and its high photocatalytic activity [J]. Journal of Materials Chemistry, 2010, 20: 5641-5645.
[20] KATO S, UNUMA H, OTA T, TAKAHASHI M. Homogeneous precipitation of hydrous tin oxide powders at room temperature using enzymatically induced gluconic acid as a precipitant [J]. Journal of the American Ceramic Society, 2000, 83(4): 986-988.
从铜合金渣中选择性回收锡及其热处理合成SnO2
Jung-Il LEE1, Jong Bum PARK1, Tae Wan KIM1, Man-Sik KONG2, Jeong Ho RYU1
1. Department of Materials Science and Engineering, Korea National University of Transportation, Chungju, Chungbuk 380-702, Korea;
2. Functional Materials Research Team, Advanced Materials & Processing Center, Institute for Advanced Engineering, Yongin, Gyunggi 449-863, Korea
摘 要:采用选择性溶解方法对铜合金废渣中的锡进行富集和分离。使用HNO3溶液溶解铜合金废渣,渣中的锡不会溶解从而使其从中得到分离。通过离心分离生成的H2SnO3沉淀相,然后经过干燥加热生成SnO2粉末。对SnO2粉末的相组成、表面形貌和化学成分进行分析。
关键词:回收锡;铜合金渣;选择性溶解;二氧化锡
(Edited by Sai-qian YUAN)
Corresponding author: Jeong Ho RYU; Tel: +82-43-841-5384; Fax: +82-43-841-5380; E-mail: jhryu@ut.ac.kr
DOI: 10.1016/S1003-6326(14)63304-5