
Density of liquid NiCoAlCr quarternary alloys
measured by modified sessile drop method
FANG Liang(方 亮)1, ZHANG Shu-fang(张淑芳)1, XIAO Feng(肖 锋)2, YANG Ling-chuan(杨凌川)1,
DONG Jian-xin(董建新)1, CAO Chun-lan(曹春兰)3, TAO Zai-nan(陶再南)4, K. MUKAI4
1. Department of Applied Physics, Chongqing University, Chongqing 400044, China;
2. Chongqing Institute of Technology, Chongqing 400050, China;
3. Department of Physics, Chongqing Communication Institute, Chongqing 400035, China;
4. Satellite Venture Business Laboratory, Kyushu Institute of Technology, Kitakyushu 804-8550, Japan
Received 28 July 2006; accepted 15 September 2006
Abstract: The densities of liquid NiCoAlCr quaternary alloys with a fixed molar ratio of Ni to Co to Al (x(Ni)?x(Co)?x(Al)≈73?12?15) which is close to the average value of the commercial Ni-based superalloys TMS75, INCO713, CM247LC and CMSX-4, and the mass fraction of chromium changes from 0 to 9% were measured by a modified sessile drop method. It is found that with increasing temperature and chromium concentration in the alloys, the densities of the liquid NiCoAlCr quaternary alloys decrease, whereas the molar volume of the liquid NiCoAlCr quaternary alloys increases. And the liquid densities of NiCoAlCr quaternary alloys calculated from the partial molar volumes of nickel, cobalt, aluminum and chromium in the corresponding Ni-bases binary alloys are in good agreement with the experimental ones, i.e. within the error tolerance range the densities of the liquid Ni-based multi-component alloys can be predicted from the partial volumes of elements in Ni-based binary alloys in liquid state. The molar volume of liquid NiCoAlCr binary alloy shows a negative deviation from the ideal linear mixing and the deviation changes small with the increase of chromium concentration at the same temperature.
Key words: NiCoAlCr alloy; liquid density; liquid state; sessile drop method; molar volume
1 Introduction
Ni-based heat-resistant superalloys are widely applied to make critical components in gas-turbine engines, such as blade etc. The density(ρ) and its temperature dependence(dρ/dT) are important thermo- physical properties for the simulation of solidification and flow behavior in the casting process of alloys, i.e. prediction of the defects such as microsegregation and gas porosity.
In order to predict the density of the liquid commercial Ni-based superalloys with arbitrary chemical composition, which usually contain multi-components, such as Ni, Cr, Co, Al,W, Ta, Mo, Re, Hf, Nb, Ti, etc, it is necessary to know the effect of various elements on the densities of liquid nickel alloys. But for some alloy elements, such as Re and Hf, their concentrations in the Ni-based superalloys are relatively low but they are very reactive, making it difficult to measure the density of their Ni-based binary alloys precisely. The effect of these elements on the densities of the Ni-based superalloys can be obtained by comparing the densities of liquid Ni-based commercial alloys with those of the liquid Ni-based multi-component model alloys with similar chemical compositions.
In our previous work, the densities of liquid nickel and liquid Ni-Cr, Ni-Co, Ni-Ta, Ni-W, Ni-Al and Ni-Mo binary alloys were measured precisely by a modified sessile drop method (MSDM) and the molar volume of nickel and the partial molar volume of Cr, Co, Al, W, Ta, Mo were worked out [1-9]. However, up to now, there are few reports on the densities of Ni-based ternary and quaternary model alloys, only our group measured the densities of the liquid NiCoAl ternary and NiCrAlMo quaternary alloys [10-12].
In the present work, the densities of the liquid NiCoAlCr quarternary model alloys with a molar ratio of Ni to Co to Al close to the average value of commercial Ni-based superalloys were measured by MSDM, then the measured densities were compared with those calculated from the partial molar volumes of nickel, cobalt, aluminum and chromium in the corresponding liquid Ni-based binary alloys, and whether the liquid NiCoAlCr alloy is an ideal mixtures was discussed.
2 Experimental
The principle of MSDM is shown in Fig.1 and the experimental procedure is introduced in detail in Ref.[1]. At room temperature, the high purity nickel disc and the high purity Ni(80%)-x(20%)(x=Co, Al, Cr) master alloy discs with optimum mass are charged into a horizontal alumina crucible. When the temperature of the furnace is high enough to melt these alloys, a drop with smooth surface is formed above the upper end of the crucible. From the photos of the liquid drop, the volume of the sample can be calculated, then the density of alloy ρ, g/cm3, can be obtained by
ρ=m/V=m/(V1+V2) (1)
where m is the sample mass, g; V1 is the inner volume of crucible at experimental temperature and V2 is the volume of sample formed above the upper end of the crucible, cm3.
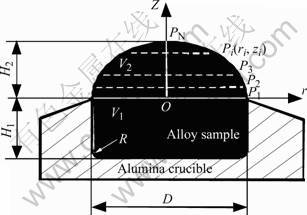
Fig.1 Schematic diagram of modified sessile drop method for measurement of density
The experimental apparatus consists of a LaCrO3 heating furnace, a gas purifier, an oxygen sensor, a photographic system and a digital system, the photos of the liquid drop are taken by the camera. The procedures were described in detail in Ref.[1].
After experiments, the chemical compositions of the alloys are analyzed by a radio frequency inductively coupled plasma (ICP) emission spectrometry and oxygen concentration in the alloy is obtained by the oxygen and sulfur analyzer. The total maximum relative error for the method in this study is estimated as ±0.75%[1].
3 Results and discussion
The chemical compositions of the samples in this work are shown in Table 1. The molar ratio of nickel to cobalt to aluminum (x(Ni)?x(Co)?x(Al)) in the quaternary NiCoAlCr alloys is fixed approximately as 73?12?15, which is close to the average value of commercial superalloys: TMS75, INCO713, CM247LC and CMSX-4, and the molar fractions of chromium change from 0 to 8.9% (mass fraction from 0 to 8.58%).
3.1 Density and molar volume of liquid NiCoAlCr alloy
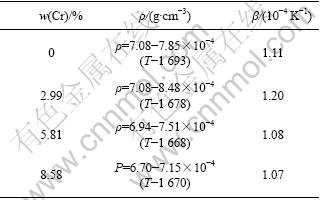
As shown in Fig.2, the densities of NiCoAlCr liquid alloys decrease linearly with increasing temperature, as described in Table 2. The volume thermal expansion coefficient, β=(dV/dT)/V=-(dρ /dT)/ρ, for the liquid NiCoAlCr alloys with various Cr concentrations exists in the range of 1.07×10-4-1.20×10-4 K-1.
The effect of chromium concentration on the densities of liquid NiCoAlCr alloys at various temper- atures is shown in Fig.3. The densities of NiCoAlCr liquid alloys decrease with increasing Cr concentration, and can be described as a quadratic function of Cr concentration (Table 3).
The temperature coefficient of the density, k=dρ/dT, of the liquid NiCoAlCr alloy changes nonlinearly with increasing chromium concentration, and can be described as a quadratic function of Cr concentration as follows by using the least-square analysis:
k×104=-7.95+0.165w(Cr)+3.16×10-2 w2(Cr) (2)
Table 1 Compositions of experimented NiCoAlCr alloys

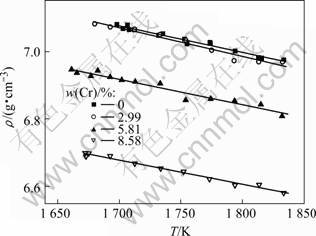
Fig.2 Temperature dependence on density of liquid NiCoAlCr alloy with different mass fractions of Cr
Table 2 Density of liquid NiCoAlCr alloys as function of temperature at different Cr concentrations
Therefore, a least-square analysis of the data gives an equation for the density of liquid NiCoAlCr alloy as a function of temperature and chromium concentration as follows:
ρ=7.08+2.08×10-2 w(Cr)-7.63×10-3 w2(Cr)-
7.95+0.165w(Cr)-3.16×10-2 w2(Cr))×10-4(T-TL)
(x(Ni)? x(Co)? x(Al)≈73?12?15, TL≤T≤1 833 K) (3)
where TL is the liquidus temperature of NiCoAlCr alloy.
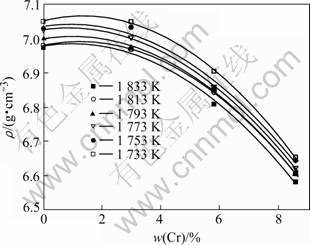
Fig.3 Effect of chromium concentration on density of liquid NiCoAlCr alloy
The calculated values from Eqn.(3) are compared with the observed data, as shown in Fig.4, the calculated data are in good agreement with the measured results in present work and the maximum difference between them is about ±0.29%.
The molar volume of liquid NiCoAlCr alloy can be calculated from the molar mass and the density data of liquid alloy. The isothermal molar volumes of NiCoAlCr liquid alloys at different temperatures increase with increasing Cr concentration, as shown in Fig.5, and described as a quadratic function of Cr concentration in Table 3. According to these equations, the partial molar volume of chromium in NiCoAlCr alloy, cm3/mol, can be calculated as follows. The way to obtain the partial molar volume can be referred to Ref.[6] as
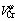 =2.77+1.43×10-3T+(140.24-3.47×10-2T) x(Cr)(4)
=2.77+1.43×10-3T+(140.24-3.47×10-2T) x(Cr)(4)
3.2 Comparison with predicted density and ideal mixing
In our previous work, the partial molar volumes of nickel, cobalt, aluminum and chromium, cm3/mol, in the corresponding Ni-based binary liquid alloys have been obtained from the measured liquid densities as follows[2-5]:
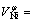 7.43+1.42×10-3×(T-1 728) (5)
7.43+1.42×10-3×(T-1 728) (5)
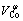 ≈3.64+1.81×10-3T (6)
≈3.64+1.81×10-3T (6)
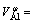 4.33+2.76×10-3T (7)
4.33+2.76×10-3T (7)
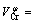 5.91+2.11×10-3T (8)
5.91+2.11×10-3T (8)
Table 3 Density and molar volume of liquid NiCoAlCr alloys at different temperatures

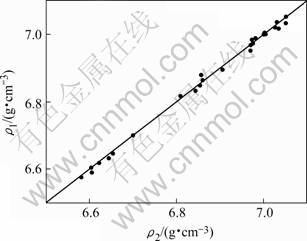
Fig.4 Comparison of measured values (ρ2) with calculated values (ρ1) for density of liquid NiCoAlCr alloy
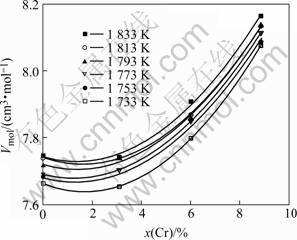
Fig.5 Relationship between molar volume and chromium concentration in NiCoAlCr alloy
With these partial molar volumes, the density of NiCoAlCr alloys can be predicted by
ρ=∑(xiMi/)/∑(xiVi) (∑xi=1, i= Ni, Co, Al, Cr) (9)
where xi, Mi, Vi are the molar ratio, atom mass and the partial molar volume of component i, respectively.
The calculated densities from Eqn.(9) are in accord with the measured results and the maximum difference between them is about ±1.50%, indicating that within the error tolerance range the density of liquid Ni-based multi-component alloys can be predicted from the partial volumes of elements in Ni-based binary alloys in liquid state.
In studies of alloys, interest centers on the extent of accommodation among atomic species, expressed as a deviation, ?Vmix, of the liquid solution from ideal volumetric mixing.
?Vmix = Vmol – Videal (10)
where Vmol is the molar volume calculated from the density and Videal is the molar volume in ideal volumetric mixing.
For NiCoAlCr alloy,
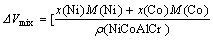 +
+
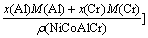 -
-
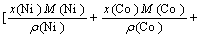
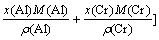 (11)
(11)
where x(Ni), x(Co), x(Al) and x(Cr), M(Ni), M(Co), M(Al) and M(Cr) and ρ(Ni), ρ(Co), ρ(Al) and ρ(Cr) are the molar fractions、molar mass and densities of pure elements of nickel, cobalt, aluminum and chromium, respectively, ρ(NiCoAlCr) is the density of NiCoAlCr liquid alloy.
Because these pure elements do not exist in the liquid state at the temperature lower than its melting point (Ni: 1 728 K; Co: 1 768 K; Al: 933 K; Co: 2 280 K), it is difficult to strictly verify the molar volume in the liquid NiCoAlCr system. However, if one assumes that the relationship can be used to calculate the density of liquid metal in metastable state at the temperature lower than the melting point, according to the density data at room temperature and at melting point [14-15], the density of these metastable metals can be calculated as follows:
Ni: ρ=7.8–7.26×10-4( T-1 728 ) (12)
Co: ρ=7.75–1.65×10-3(T-1 768) (13)
Al: ρ=2.375–2.33×10-4(T-933) (14)
Cr: ρ=6.30–1.1×10-3(T-2 280) (15)
The molar volumes of the NiCoAlCr alloy at the temperatures of 1 733 K and 1 833 K determined in the present work are shown in Fig.6. The deviation of molar volume from ideal mixing is shown in Fig.7. It shows that nickel, cobalt, aluminum and chromium mix nonideally, and a negative deviation from ideal mixing exists, implying an aggregation between nickel, cobalt, aluminum and chromium atoms. At a same chromium concentration, the deviation is almost the same at 1 733 K and 1 833 K, which means the temperature does not change the deviation so much. But at a same temperature, the deviation becomes smaller with the increase of chromium concentration, which indicates that nickel, cobalt, aluminum and chromium atoms have a trend to mix ideally at higher chromium concentration.
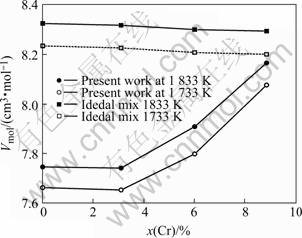
Fig.6 Comparison of measured molar volume with that in ideal mixture
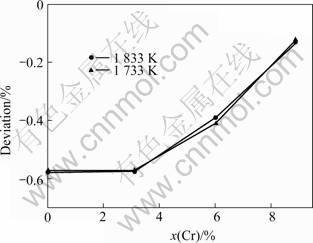
Fig.7 Deviation of molar volume from ideal mixing in NiCoAlCr alloy
4 Conclusions
1) The densities of liquid NiCoAlCr alloy were measured by a modified sessile drop method. The density of liquid NiCoAlCr alloy decreases with increasing temperature and chromium concentration in the alloys.
2) The density of the liquid NiCoAlCr alloy as functions of both temperature and chromium concentration was expressed. The calculated values show good agreement with the measured values. The maximum difference is about ±0.29%.
3) The molar volume of liquid NiCoAlCr binary alloy increases with the increase of temperature and chromium concentration, and shows a negative deviation from the ideal linear mixing molar volume. The deviation changes smaller with the increase of chromium concentration at the same temperature.
4) The density of liquid NiCoAlCr quarternary alloys can be predicted from the partial volumes of elements in Ni-based binary alloys in liquid state.
References
[1] MUKAI K, XIAO Feng. Density of Ni-Cr alloy in liquid and solid-liquid coexistence states [J]. Mater Trans, JIM, 2002, 43(5): 1153-1160.
[2] FANG Liang, XIAO Feng, WANG Yin-feng, TAO Zai-nan, MUKAI K. Density and molar volume of liquid Ni-Co binary alloys[J]. Mater Sci Eng B, 2006, B132(1/2): 174-178.
[3] FANG Liang, XIAO Feng, LI Zu-shu, TAO Zai-nan. Density of Ni-Al alloys in liquid state and solid-liquid coexistence state measured by a modified pycnometric method [J]. J Mater Sci Technol, 2004, 20(4): 405-410.
[4] FANG Liang, XIAO Feng, TAO Zai-nan. Density of liquid binary Ni-W alloys measured by modified sessile[J]. Rare Metal Materials and Engineering, 2004, 33(12): 1261-1265.
[5] FANG Liang, XIAO Feng, TAO Zai-nan. Density measurement of liquid Ni-Ta alloys by a modified sessile drop method [J]. J Wuhan Uni Technol, 2005, 20(2): 67-70.
[6] FANG Liang, LI Zu-shu, TAO Zai-nan, XIAO Feng. Density of liquid Ni-Mo alloys measured by a modified sessile drop method [J]. J Mater Sci Technol, 2004, 20(3): 287-292.
[7] XIAO Feng. Density of liquid Ni-Cr alloy[J]. .J Mater Sci Technol, 2003, 19 (1): 16-18.
[8] XIAO Feng, FANG Liang. Density and structure analysis of molten Ni-W alloys[J]. J Mater Sci Technol, 2004, 20(4): 410-413.
[9] MUKAI K, FANG Liang, LI Zu-shu, XIAO Feng. Measurement of the density of binary Ni-X(X=Co, W, Ta, Al) alloys[J]. Mater Trans, JIM, 2004, 45(5): 1754-1763.
[10] FANG Liang, WANG Yin-feng, XIAO Feng, TAO Zai-nan, MUKAI K. Density of liquid NiCrAlMo quarternary alloys measured by a modified sessile drop method [J]. Mater Sci Eng B, 2006, B132(1/2): 164-169.
[11] MUKAI K, LI Zu-shu, FANG Liang. Measurement of the densities of nickel-based ternary, quaternary and commercial alloys[J]. Materials Transactions, 2004,45(10): 2987-2993.
[12] XIAO Feng, FANG Liang, Measurement and analysis of molten Ni-Co-Al alloy density [J]. J Mater Sci Technol, 2003, 19(5): 388-390.
[13] BAKER H. Alloy Phase Diagrams[M]. New York: Metals Park, ASM, 1992: 135-150.
[14] LEE H. Chemical Thermodynamics for Metals and Materials[M]. London: Emperial College Press, 1999: 284-500.
[15] LIDE D R. CRC Handbook of Chemistry and Physics [M]. New York: CRC Press, 1997, 129: 4-126.
(Edited by CHEN Wei-ping)
Foundation item: Project(NCET-05-0764) supported by the Program for New Century Excellent Talent in University; project(2004527) supported by SRF for ROCS, SEM
Corresponding author: FANG Liang; Tel: +86-23-65105870; E-mail: fangliangcqu@yahoo.com.cn