
Effects of added Cd on Cd uptake by oilseed rape and pai-tsai co-cropping
LIU Yun-guo(刘云国), YE Fei(叶 菲),ZENG Guang-ming(曾光明),
FAN Ting(樊 霆), MENG Lei(孟 蕾), YUAN Hua-shan(袁华山)
College of Environmental Science and Engineering, Hunan University, Changsha 410082, China
Received 17 October 2006; accepted 18 April 2007
Abstract: The experiment was designed to study whether the decrease of Cd in the rhizosphere of Zhongyouza Ⅰ, one breed of oilseed rapes (Brassica junica) that can hyperaccumulate cadmium from the soil, can improve the living condition of less Cd-tolerant plant pai-tsai (Brassia chinensis) with their roots permitted to intermingle and develop coincident rhizosphere. The extent of rhizosphere interactions between ZhongyouzaⅠ and pai-tsai was controlled by different root barriers, or without barrier. The results show that in the 10 and 20 mg/kg Cd treated soils, pai-tsai gets higher shoot mass and less Cd accumulation in its shoot than in the barrier treatments or in the mono cultures, when its roots are permitted to intermingle with those of ZhongyouzaⅠ. Meanwhile, soil decontamination rates of ZhongyouzaⅠ are not affected much by co-cropping with pai-tsai, they are 80.0% and 91.8% of that in the mono cultures of ZhongyouzaⅠ, respectively. However, the co-cropping method in meliorating the living condition of pai-tsai is not obvious when Cd concentration in soil reaches 40 mg/kg, and soil decontamination rate decreases to 0.14, which is 58.3% of that in the mono culture. These results indicate that the oilseed rape ZhongyouzaⅠ may alleviate Cd toxicity of surrounding less-tolerant species, and its ability of phytoremediation is not affected much at the same time, especially in the middle polluted soil.
Key words: oilseed rape; pai-tsai; cadmium; co-cropping; phytoremediation
1 Introduction
Heavy metal contamination of soils originating from industry or agriculture, such as smelting industries, residues from metalliferous mined, pesticide, fertilizers, municipal compost, is one of the major environmental problems in the world. Excessive metal concentrations in the contaminated soils can result in soil quality degradation, crop yield reduction, and poor quality of agriculture products[1]. The subsequently transferred and accumulated heavy metals in human beings’ bodies through food chain might be a threat to health. In recent years, scientists have been concerned about the use of metal-accumulating plants for phytoextraction of metals from contaminated soils[2-3]. However, STEVEN et al[4] pointed out a more direct way of phytoprotection, in which the Zn hyperaccumulator plant Thlaspi caerulesccens could aid the establishment of the co-cropped species Thlaspi arvense that would find Zn in the soil to be toxic and inhibit to growth. Up to now, there has been little information about the effects of Cd uptake by the co-cropping method in Cd contaminated soil.
Cadmium is one of the most toxic heavy metals that are widespread pollutants of the surface soil layer. It inhibits root and shoot growth, affects nutrient up-take and homeostasis, and is frequently accumulated by agriculturally important crops[5-6]. In the remediation of Cd polluted soils, the Cd hyperaccumulator plant, Indian mustard, is often used. However, a few researches show that some breeds of oilseed rapes (Brassica junica), such as Xikouhuazi, ChuanyouⅡ, Zhongyou119, have even higher hyperaccumulating ability than Indian mustard[7-9], and ZhongyouzaⅠ is the one that shows the most outstanding ability among them. It presents a more excellent phytoremediation potential when comparing shoot biomass, content of Cd, Cd uptake amount and removal rate in Cd contaminated soil with Indian mustard[7].
ZhongyouzaⅠwas chosen as the Cd accumulator in test if there was a positive effect on the growth of the co-cropped less tolerant species. We did this to know that how a crop species could be cropped in the Cd polluted soil, hoping that the agriculture production could carry on together with phytoremediation. The research was designed to investigate the effects of three levels of root interaction and four levels of Cd concentration on the shoot mass and Cd accumulated by ZhongyouzaⅠand the less tolerant plant cropped in mono- or mixed- cultures. The extent of root interaction between the hyperaccumulator and the nonaccumulator plants was controlled by vertical root barriers (no barrier, nylon mesh barrier or plastic sheet barrier). Cd concentration in the soils varied from 0 to 40 mg/kg. Pai-tsai (Brassia chinensis) that had a much lower threshold of tolerance to Cd than ZhongyouzaⅠwas chosen as the companion species.
2 Material and methods
2.1 Construction of pots
The PVC (polyvinyl chloride) pots of 15 cm in height and 12 cm in diameter were prepared in there types: one was undivided (no root barrier), permitting complete root intermingling; the second pot was intermediate, with a vertical nylon mesh root barrier (35 ?m pore size) to separate roots, but soil solution exchange is permitted; the last pot was completely divided into two halves by a vertical barrier of plastic sheet, so the root and soil interactions between the two compartments were impermeable. 36 pots of each type were prepared before the experiment.
2.2 Cd enrichment of soil
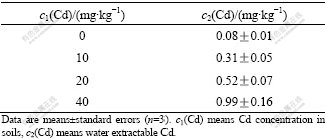
The red soil sample used in this study was collected from an unpolluted site of Yuelu Mountain, China. Total Cd content in the soil was 0.10 mg/kg. The soil was subsequently air-dried, ground, screened through a 2 mm sieve to remove large particles, and divided into 4 parts. The separated soils were then enriched to 4 different nominal concentrations (0, 10, 20 and 40 mg/kg soil) respectively in the form of CdCl2. 1 kg of each concentration CdCl2- treated soils was added to each of 27 pots (9 pots of each type), which is a quarter of the total. As the water-soluble forms of metals are considered to be the most available to plants[10], extraction with water was used as an estimate of the concentration of soluble-Cd in the soils. Water- extractable Cd of the soils was measured after equilibration for 14 d in a controlled environmental room (16 h, 20 ℃ in day, 8 h, 14 ℃ at night) at 60% water holding capacity (WHC) (Table 1). pH value of soil sample (pH=5.87) was measured in a mixture with mass ratio of soil to water of 1?2.5.
Table 1 Water-extractable Cd concentration in different Cd concentration soils
2.3 Plants co-cropping
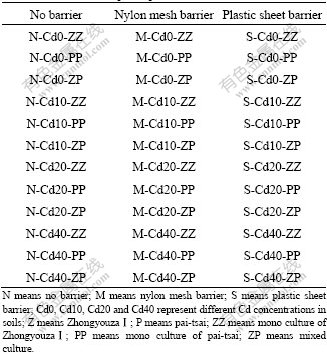
Nine treatments of each Cd concentration soils were prepared for the study (Table 2). Ten seedlings of ZhongyouzaⅠwere planted into each half of the pots as indicated by “Z” in Table 2. Seven days later, when the shoot height of ZhongyouzaⅠwas about 2 cm, ten seedlings of the less tolerant plant pai-tsai (breeding in a greenhouse with unpolluted soil, shoot height was also about 2 cm) were transplanted to each side of the pots indicated by “P” in Table 2. This gave either a monoculture of ZhongyouzaⅠ, a monoculture of pai-tsai, or a mixed culture of ZhongyouzaⅠon one side of the pot and pai-tsai on the other. And there was 36 different treatments in total (Table 3). Each treatment was replicated three times. The samples were randomized in the controlled environment room and deionized water was added to return the soils to 60% WHC as required. Forty-two days after the ZhongyouzaⅠwas transplanted into the pots, shoots on each side of the pots were harvested and dried at 70 ℃ for determination of shoot dry mass and concentration of Cd. The dry shoots were weighed and then digested by HNO3-HClO4 method. Cd concentrations in the digested solutions were determined by atomic absorption spectroscopy(AAS).
Table 2 Nine treatments of co-planted pai-tsai and ZhongyouzaⅠdesigned for experiment (P is pai-tsai (less tolerant species); Z is ZhongyouzaⅠ(hyperaccumulator))
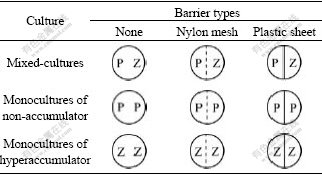
Table 3 Treatments of pot experiment
2.4 Statistical analyses
In order to compare data difference between the treatments (i.e. mixed- or mono- culture, the extend root intermingling permitted, and different Cd concentrations in soils) on shoot mass, Cd concentration, and mass of Cd accumulated in each species, statistical analysis system(SAS) was taken into analysis. Inspection of the data sets indicated that a few failed the requirement of SAS. Least significant difference was used to compare the effects at p<0.05. Significance SAS were shown by letter codes. Values with the some letter in figures were not significantly different.
3 Results and discussion
3.1 Shoot mass
Although the shoot mass of the plants in all the Cd enriched soil treatments decreases compared with that of the plants in 0 mg/kg Cd treatment (Figs.1 and 2), significant increases are still found in the growth of both species when unrestricted root intermingling is permitted between ZhongyouzaⅠand pai-tsai. By taking 10 mg/kg Cd treatment into consideration, the shoots of pai-tsai attain the same biomass irrespective of the extending of root intermingling permitted in the pots in monocultures (Fig.3(a), black bars). However, when grown in mixed culture with ZhongyouzaⅠ(hatched bars), the shoot biomass of pai-tsai is increased by 47.8% when there is no barrier to prevent the root intermingling (Fig.3(a), p<0.05) and 22.9% in mesh barrier treatment where soil solution exchange is permitted only (Fig.3(a); not significant, p>0.05), compared with that in the plastic sheet treatment. The hyperaccumulator ZhongyouzaⅠshows nearly the same growth complexion in this research. Its shoot mass remains constant in monoculture, irrespective of the extending of root intermingling permitted (Fig.4(a), black bars). Similarly, shoot biomass of ZhongyouzaⅠ also remains unaffected when the roots intermingling of the two species are separated by plastic sheet or nylon mesh. However, when grown in the mixed culture with pai-tsai (hatched bars), the shoot mass of ZhongyouzaⅠis increased by 24.8% in the pots without barriers (p<0.05). These positive effects on the shoot mass of ZhongyouzaⅠwhen sharing its rhizosphere with pai-tsai suggest that greater intensity of competition in monoculture than that grown at the same density in mixed culture, where ZhongyouzaⅠ benefits from reduced competition for resources in the pot. The increased growth of pai-tsai when sharing a rhizospere with ZhongyouzaⅠmay follow the same mechanism in mixed culture. However, the contrast of pai-tsai’s shoot mass by 0-10 mg/kg Cd treatment shows clearly that this effect is different in these soils. In 0 mg/kg Cd treatment where Cd is not enriched to be a threat to the growth of pai-tsai, the plants of pai-tsai attain a shoot biomass of about 1 700 mg,which is nearly twice of that in monoculture of 10 mg/kg Cd treatment. It is obvious that high concentration of Cd in the soil prevents the growth of pai-tsai, and this plant will not show highly competitive abilities when suffering from such Cd toxicity. This indicates that the enhanced shoot mass of nonaccumulator pai-tsai should owe to the decrease of Cd toxic effects by co-cropping with Cd hyperaccumu- lator plant ZhongyouzaⅠ.
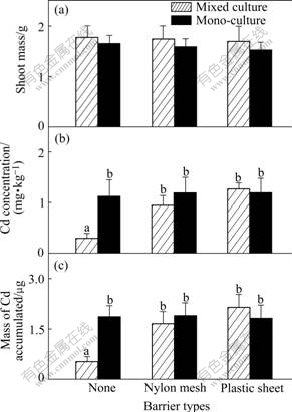
Fig.1 Shoot mass of P (a), Cd concentration (b) and total mass of Cd accumulated (c) in shoot per pot of pai-tsai in 0 mg/kg Cd treated soil (Hatched bars: Data from mixed cultures; Black bars: Data from one side of the monocultures. Three types of pots are indicated using symbols shown in Table 2. Data are means+ standard errors, n=3)
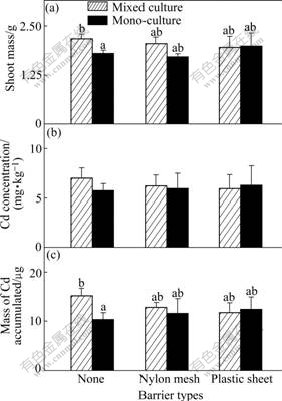
Fig.2 Shoot mass of Z (a), Cd concentration (b) and total Cd accumulated (c) in shoot per pot of ZhongyouzaⅠ in 0 mg/kg Cd treated soil (Hatched bars: Data from mixed cultures; Black bars: Data from one side of monocultures. Three types of pots are indicated using symbols shown in Table 2. Data are means+ standard errors, n=3)
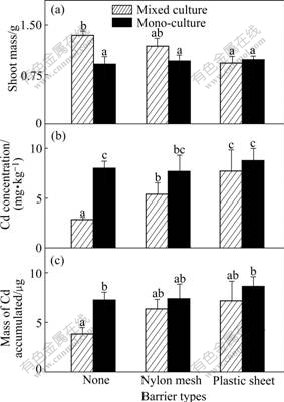
Fig.3 Shoot mass of P (a), Cd concentration (b), and total Cd accumulated (c) in shoot per pot of pai-tsai in 10 mg/kg Cd treated soil (Hatched bars: Data from mixed cultures; Black bars: Data from one side of monocultures. Three types of pots are indicated using symbols shown in Table 2. Data are means+ standard errors, n=3)
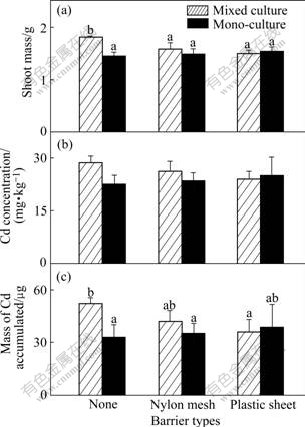
Fig.4 Shoot mass of Z (a), Cd concentration (b) and total Cd accumulated (c) in shoot per pot of ZhongyouzaⅠin 10 mg/kg Cd treated soil (Hatched bars: Data from mixed cultures; Black bars: Data from one side of monocultures. Three types of pots are indicated using symbols shown in Table 2. Data are means+ standard errors, n=3)
3.2 Cd concentration in shoot
The increased growth of pai-tsai is due to the reduced concentrations of Cd in the shoots when its roots are permitted to intermingle with those of Zhongyouza Ⅰ. In 10 mg/kg Cd treatment, it can be seen constant values of the Cd concentrations in the shoots of the two plants in monocultures, irrespective of the extent of root intermingling permitted (Fig.3(b), black bars; Fig.4(b), black bars). However, pai-tsai gets a low Cd concentration of 2.8 mg/kg in the mixed culture with ZhongyouzaⅠ(Fig.3(b)) in the pots without barrier, which is 1/3 of that in monocultures (p<0.05=, and 5.4 mg/kg in the mixed culture treatment with nylon mesh barrier. Furthermore, the total Cd accumulated by pai-tsai in shoots is the lowest in the treatment where complete root intermingling is permitted with ZhongyouzaⅠ (Fig.3(c)) among these treatments. Increasing Cd concentration and Cd accumulation in shoots of ZhongyouzaⅠ when sharing its rhizosphere with pai-tsai (Figs.4(b) and (c), hatched bars) gives another confirmation that its ability of accumulating can aid the growth of the less tolerant species plant pai-tsai.
3.3 Growth complexion in other Cd treated soils
The growth responses of the two plants in 20 mg/kg Cd treatment are nearly the same as those in 10 mg/kg Cd treatment. They both get the highest shoot mass when their roots are permitted to intermingle (Fig.5(a) and Fig.6(a)). Similarly, Cd concentration and Cd accumulated in the shoot of pai-tsai are also less than those in the other treatments when sharing a full root interaction with the hyperaccumulator (Fig.5(b), p<0.05; Fig.5(c), not significant, p>0.05). However, Completely similar rules cannot be found in 40 mg/kg Cd treatment. The hyperaccumulator ZhongyouzaⅠ actually shows less shoot mass when its root intermingles completely with pai-tsai than in other treatments (Fig.7(a); not significant, p>0.05), and this value even does not reach a half of that in the 20 mg/kg Cd treated soil, though the concentration of Cd in shoot is the greatest in this treatment (Fig.7(b); not significant, p>0.05). Meanwhile, poor shoot mass results in decreasing of Cd accumulation. The less tolerant plant pai-tsai increases little in its shoot mass, which is not significant (Fig.8(a), p>0.05) when full root interaction is permitted with ZhongyouzaⅠ.
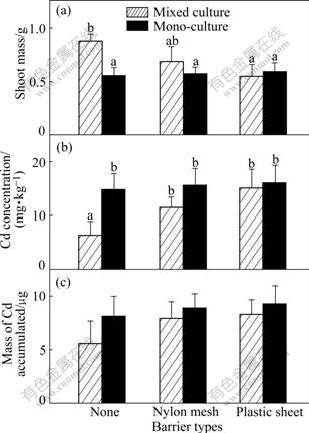
Fig.5 Shoot mass of P (a), Cd concentration (b) and total Cd accumulated (c) in shoot per pot of pai-tsai in 20 mg/kg Cd treated soil (Hatched bars: Data from mixed cultures; Black bars: Data from one side of monocultures. Three types of pots are indicated using the symbols shown in Table 2. Data are means+ standard errors, n=3)
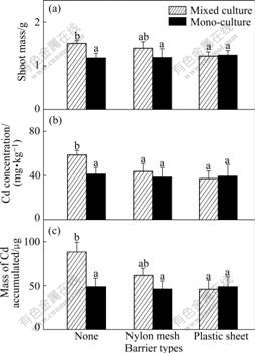
Fig.6 Shoot mass of Z (a), Cd concentration (b) and total Cd accumulated (c) in shoot per pot of ZhongyouzaⅠin 20 mg/kg Cd treated soil (Hatched bars: Data from mixed cultures; Black bars: Data from one side of monocultures. Three types of pots are indicated using symbols shown in Table 2. Data are means+ standard errors, n=3)
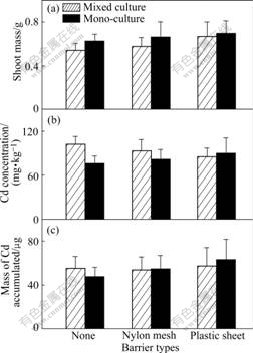
Fig.7 Shoot mass of Z (a), Cd concentration (b) and total Cd accumulated (c) in shoot per pot of ZhongyouzaⅠin 40 mg/kg Cd treated soil (Hatched bars: Data from mixed cultures. Black bars: Data from one side of monocultures. Three types of pots are indicated using symbols shown in Table 2. Data are means+ standard errors, n=3)
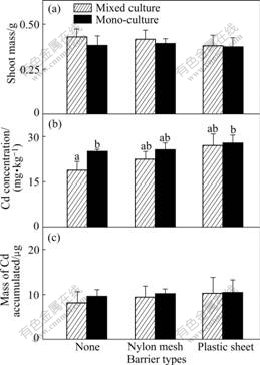
Fig.8 Shoot mass of P (a), Cd concentration (b) and total Cd accumulated (c) in shoot per pot of pai-tsai in 40 mg/kg Cd treated soil (Hatched bars: Data from mixed cultures. Black bars: Data from one side of monocultures. Three types of pots are indicated using symbols shown in Table 2. Data are means+ standard errors, n=3)
Furthermore, in the mixed culture of 40 mg/kg Cd treatment without barrier, Cd concentration in shoot of pai-tsai is more than 6 times of that in 10 mg/kg Cd treatment, and 3 times of that in 20 mg/kg Cd treatment. However, these similar relations are not found in the comparison of water-soluble Cd in soils or in the monocultrues of different Cd treated soils. High Cd concentration in soil restricts the growth of ZhongyouzaⅠand causes less protection by it on the nonaccumulator species pai-tsai, even with their roots intermingled.
Recently, some other factors such as calcium or zinc [11-12], rare earth[13], and organic manure[14], have been proved to be efficient in enhancing heavy metal tolerance and reducing the amount of heavy metals accumulated by plants. The suppose of adding the available factors into co-planting structure may enhance the accumulation of fewer heavy metals by less tolerant crops, need to be proved in further studies.
3.4 Comparason of decontamination rate between mixed- and mono-cultures
Ratio of the mass that one heavy metal accumulated in a shoot of plant to the total that contained in the soil, is called decontamination rate(DR). It is an important guide line to estimate the efficiency of a plant in remediating heavy metal polluted soil. As treatments with no barriers show the most significant effects on protecting pai-tsai, and may be used in practical production, this type of treatment is taken into discussion here. Total Cd in the soils was decided by how much we put in, for background value of Cd is low in soil and can be neglected. The soil decontamination rate of ZhongyouzaⅠis calculated when a pot contains the hyperaccumu- lator. The results are listed in Table 4.
Table 4 Decontamination rate under different treatments (%)
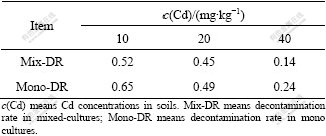
By comparing the data in Table 4, it is found that the decontamination rate decreases with increasing concentration of Cd in soils in both mixed and mono- cultures. However, in 10 mg/kg Cd treated soil, the decontamination rate is 0.52 in mixed-culture, which is 80.0% of that in mono-culture of ZhongyouzaⅠ. Furthermore, the same decontamination rate in the mixed and the mono-cultures is got when Cd concentration in soil is 20 mg/kg. These indicate that the phyto- remediation ability of ZhongyouzaⅠ on heavy metal polluted soil is affected little when it alleviates the Cd toxicity of pai-tsai in the mixed culture in 10 and 20 mg/kg Cd treated soils, but not including in the 40 mg/kg treatment, whose decontamination rate in mixed culture is only 58.3% of that in the mono-culture.
4 Conclusions
1) In the middle Cd polluted soils (10, 20 mg/kg Cd), ZhongyouzaⅠthat shares a full root interaction with pai-tsai, gets more advantages in enhancing the growth and reducing Cd concentration in shoot of the less tolerant plant than in other barrier treatments. Such influence should be considered in the strong Cd accumulation of the hyperaccumulator. However, the protection ability of ZhongyouzaⅠis not significant when Cd concentration in the soil is high (40 mg/kg Cd), due to the sharp decrease in shoot mass and Cd accumulated in shoot when the hyperaccumulator suffering from such high toxicity.
2) The decontamination rate is affected little in the mixed culture when ZhongyouzaⅠmeliorating the living condition of pai-tsai in middle polluted soils. Therefore, the normal growth of pai-tsai and the soil remendiation can go along at the same time. But co-cropping of the two plants decreases the ability of soil remendiation in 40 mg/kg Cd treatment.
3) In the present study, the hyperaccumulator is precropped to phytoextract the soluble or toxic fraction of metal from the soil before planting a crop species. The co-planted crop is protected from metal toxicity. However, this will take more time to the revegetation operation and not so efficient in practical use. The effects of concurrently establishing a mixed culture of hyperaccumulators with less tolerant crops also deserve further investigation.
References
[1] JANG L Y, YANG X E, HE Z L. Growth response and phytoextraction of copper at different levels in soils by Elsholtzia splendens [J]. Chemosphere, 2004, 55: 1179-1187.
[2] RICHARD B M. Phytoremediation of toxic elemental and organic pollutants [J]. Current Opinion Biology, 2000, 3(2): 153-162.
[3] KHAN A G, KUEK C, CHAUDHRY T M, KHOO C S, HAYES W J. Role of plants, mycorrhizae and phytochelators in heavy metal contaminated land remediation [J]. Chemosphere, 2000, 41(1/2): 197-207.
[4] STEVEN N W, JONATHAN R L, STEVE P M, ALAN J M B. Hyperaccumulation of Zn by Thlaspi caerulescens can ameliorate Zn toxicity in the rhizosphere of cocropped Thlaspi arvense [J]. Environmental Science and Technology, 2001, 35: 3237-3241.
[5] SANIT? D T, GABRIELLI R. Response to cadmium in higher plants [J]. Environmental and Experimental Botany, 1999, 41: 105-130.
[6] BELIMOV A A, HONTZEAS N, SAFRONOVA V I, DEMCHINSKAYA S V, PILUZZA G, BULLITTA S, GLICK B R. Cadmium-tolerant plant growth-promoting bacteria associated with the roots of Indian mustard(Brassica juncea L.Czern) [J]. Soil Biology and Biochemistry, 2005, 37: 241-250.
[7] WANG Ji-qing, LIU Bo, SU De-chun. Selection of oilseed rapes as a hyperaccumulator for cadmium [J]. Journal of Agriculture University of Hebei, 2003, 26: 13-16. (in Chinese)
[8] WANG Ji-qing, RU Shu-hua, SU De-chun. Selection of a hyperaccumulator for phytoremediation of cadmium contaminated soil [J]. Journal of China Agricultural University, 2003, 8(1): 67-70. (in Chinese)
[9] SU De-chun, HUANG Huan-zhong. The phytoremediation potential of oilseed rape (B. juncea) as a hyperaccumulator for cadmium contaminated soil [J]. China Environmental Science, 2002, 22(1): 48-51. (in Chinese)
[10] PETRUZZELLI G. Recycling waste in agriculture: Heavy metal bioavailability [J]. Agriculture, Ecosystems and Environment, 1989, 27: 493-503.
[11] HE Z Y, LI J C, ZHANG H Y, MA M. Different effects of calcium and lanthanum on the expression of phytochelatin synthase gene and cadmium absorption in Lactuca sativa [J]. Plant Science, 2005, 168: 309-318.
[12] HE P P, L? X Z, WANG G Y. Effects of Se and Zn supplementation on the antagonism against Pb and Cd in vegetables [J]. Environment International, 2004, 30: 167-172.
[13] XING Yong, JIA Peng, ZHOU Ya-jun. Effects of rare earth on seed vigor and rice anti-heavy metal pollution [J]. Chinese Rare Earth, 2004, 25: 46-48. (in Chinese)
[14] HUA Luo, BAI Ling-yu, WEI Dong-bao, CHEN Shi-bao. Combination of pollutants cadmium and zinc and its effects on Cd accumulation in wheat grain and adjustment by organic manure [J]. Agro-environmental Protection, 2002, 21(5): 393-398. (in Chinese)
Foundation item: Project(04JJ3013) supported by the Natural Science Foundation of Hunan Province, China; Project(2001AA644020) supported by the National High-Tech Research and Development Program of China
Corresponding author: LIU Yun-guo; Tel: +86-731-8649208; E-mail: Liuyunguo@hnu.cn
(Edited by CHEN Wei-ping)