
Growth and surface properties of new thermoacidophilic Archaea strain Acidianus manzaensis YN-25 grown on different substrates
HE Huan(何 环), YANG Yi(杨 益), XIA Jin-lan(夏金兰),
DING Jian-nan(丁建南), ZHAO Xiao-juan(赵小娟), NIE Zhen-yuan(聂珍媛)
Key Laboratory of Biometallurgy of Ministry of Education,
School of Minerals Processing and Bioengineering, Central South University, Changsha 410083, China
Received 20 September 2008; accepted 5 November 2008
Abstract: The growth and surface properties of new thermoacidophilic Archaea strain Acidianus manzaensis YN-25 isolated from an acid hot spring in Tengchong, Yunnan Province, China were investigated cultured on different substrates including soluble substrate ferrous sulfate and nonsoluble solid substrates S0, pyrite and chalcopyrite. The growth characteristics of the cells in each substrate were characterized with the changes in cell number, pH, Eh, and concentrations of Fe2+ or SO42-, or ratios of [Fe2+] to [Fe3+], and the surface properties were characterized and analyzed in terms of Zeta-potential, hydrophobicity, and surface FT-IR spectra of the cells. The results show that the cells grown on solid substrates have higher value of isoelectric points. They are more hydrophobic and express more surface proteins than ferrous sulfate grown cells.
Key words: surface chemistry; Acidianus manzaensis; bioleaching; extracellular polymers
1 Introduction
It is well known that bioleaching of metal sulfides is an interfacial process comprising the interactions of adhered bacterial cells and bacterial extracellular polymeric substances(EPS) with the mineral surface [1-2]. The primary adhesion of bacterial cells to the mineral surface depends on not only the interfacial process between the cells and mineral surface but also the biochemical properties of cells. Furthermore, it has been reported that the surface properties of bacterial cells depend on the growth conditions[3-5]. Hence, a better understanding of the surface properties of leaching bacteria under different conditions is helpful to illustrating the interfacial process between cells and mineral surface[6-7].
The extremely thermophilic microbes have become the research hotspot in the recent years because of their special living conditions, physicochemical characteristics and excellent leaching capability of metal sulfides (e.g. a recovery above 90% of copper from chalcopyrite has been achieved by acidophilic thermophile Acidianus brierleyi[8-10]). Nevertheless, compared with the knowledge about the interfacial process of mesophilic bacteria such as A. ferrooxidans, only a few researches were referred to extremely thermophilic microbes, such as the typical archaeal genera Acidianus, Metallosphaera and Sulfolobus[11-12].
A. manzaensis is a novel thermoacidophilic Archaea of Acidianus genus, firstly isolated from a hot fumarole in Manza, Japan[13]. The strain A. manzaensis YN-25 was just isolated from an acid hot spring in Tengchong, Yunnan Province of Southwestern China. Bioleaching experiment for chalcopyrite indicated it had excellent leaching ability, with a copper leaching ratio up to 79.16% after 24 d. In the present work, the surface chemistry of A. manzaensis cells grown under different conditions was studied.
2 Experimental
2.1 Strain and culture condition
The strain A. manzaensis YN-25 was isolated from an acid hot spring sample in Tengchong, Yunnan Province, China. It was cultured at 65 ℃ in the basal mineral salts medium (JCM 9191T) added with (per liter) FeSO4?7H2O 35 g, elemental sulfur 10 g, pyrite 30 g and chalcopyrite 30 g, with the initial pH of 1.5, 2.5, 1.5 and 1.5, respectively.
The floated grade pyrite and chalcopyrite were provided by Institute of Mineral Processing Engineering, School of Resources Processing and Bioengineering, Central South University, China. The main contents of pyrite and chalcopyrite were as follows (mass fraction): pyrite (Fe 42.25%, S 48.34%, Cu 0.325%) and chalcopyrite (Cu 30.60%, Fe 22.64%, S 29.60%), and mineral powders used in the experiments had a size distribution of 95% less than 74 ?m.
2.2 Cell samples preparation
Bacterial cells were grown to later exponential phase and the cultures were filtered three times through filter paper and the filtrate was centrifuged, then the cell pellets were washed twice in sulfuric acid (0.1 mol/L) to remove any trapped ions. Finally, all the samples were freeze-dried and stored at -20 ?C for Zeta-potential and FT-IR measurements.
2.3 Zeta-potential measurements
The Zeta-potential measurements of cells were determined by Zeta Potential & Size Distribution Analyzer (Delsa 440SX) at a specified pH value. The Zeta-potential measurements were conducted on bacterial suspensions of 1.0×108 cell/mL with ionic intensity of 10-3 mol/L KCl.
2.4 Hydrophobicity measurement of cell surface
The hydrophobicity of cells in different growth period with different energy sources was determined by a modified method of ROSENBERG et al[14], SO and YOUNG[15] as follows. The cell samples were suspended with 5 mL of basal mineral salts in tubes, and then the OD600 values of mixture were recorded with spectrophotometer (UV-9200). Subsequently, an equal volume of hexadecane was added. After rotating for 90 s and settling for 30 min for phase separation, the OD600 values of samples at the bottom aqueous phase were recorded, and then the hydrophobicity was calculated.
2.5 FT-IR analysis
The FT-IR spectra of cells grown under different conditions were performed using a Fourier transform spectrometer (Nicolet Nexus 670) with diffusion reflectance attachment.
3 Results and discussion
3.1 Growth characteristics in different energy sub- strates
The growth characteristics of A. manzaensis YN-25 in ferrous ions are shown in Fig.1. The growth was proportional to the consumption of ferrous ions. Initially, the pH value increased due to the consumption of protons by the bacteria, and thereafter, it decreased due to the abiotic hydrolysis of ferric sulfate.
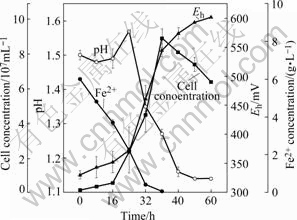
Fig.1 Growth characteristics of A. manzaensis cells grown on ferrous sulfate
The growth characteristics of cells in elemental sulfur are presented in Fig.2. In the first 36 h, the growth rate was similar with that in ferrous sulfate. However, the cell density kept on increasing till incubation for 56 h, and it was much higher than that in ferrous sulfate. With the increase of cell density, the concentration of SO42- was gradually increased, and the pH values of culture solution decreased.
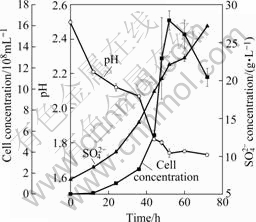
Fig.2 Growth characteristics of A. manzaensis on elemental sulfur
The growth characteristics of the cells in pyrite are shown in Fig.3. The cell number increased with incubation time. The pH of culture solution decreased continuously (finally down to 1.2 after 12 d). These results did not coincide with reported pyrite leaching mechanism of ROHWERDER et al[16]. And after that,
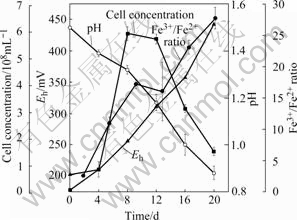
Fig.3 Growth characteristics of A. manzaensis cells grown on pyrite
the cell density came down.
The growth characteristics of cell grown on chalcopyrite are shown in Fig.4. The ratio of Fe3+/Fe2+, which was an important parameter, increased with incubation time. With the increase of incubation time, the Eh value gradually increased from 338 to 559 mV. The pH value of the culture solution initially increased slightly up to 1.6, due to the consumption of protons. As the sulfur released from chalcopyrite was oxidized to sulfuric acid, the pH of solution decreased after the 4th day.

Fig.4 Growth characteristics of A. manzaensis cells grown on chalcopyrite
3.2 Zeta potential studies
The Zeta-potentials of A.manzaensis grown on ferrous, sulfur, pyrite and chalcopyrite as a function of pH are shown in Fig.5. The A. manzaensis grown on ferrous sulfate showed an isoelectric point of pH 2.5. However, the higher isoelectric point values were observed in the sulfur-grown cells, as well as those in pyrite- and chalcopyrite-grown cells. Three solid substrate-grown cells exhibited isoelectric point at pH 3.4-3.7, among which the chalcopyrite-grown ones showed the highest isoelectric point value. The maximum negative charge occurred at the point of about pH 7.5, and the magnitudes were -25, -21, -16 and -7 mV for chalcopyrite, pyrite, sulfur and ferrous sulfate, respectively. These results show that the growth conditions significantly influence the net surface charge. And the results were similar to the finding of BLAKE et al[3], SHARMA et al[5] and NATARAJAN et al[17]. Isoelectric point is a parameter indicating the presence of functional groups such as carboxyl (—COOH), amino (—NH), and hydroxyl (—OH), which are the components of cell surface polymers and decide the surface charge of cells[18]. So the difference in surface isoelectric point indicated that the surface compounds of A. manzaensis were significantly different from the culture conditions. According to the studies on A. ferrooxidans before, the isoelectric point (pH 2.5) of ferrous ions-grown cells indicated the existence of significant amounts of glucuronic acids or other polysaccharides containing negatively charged phosphate and/or carboxyl groups. However, the isoelectric point of the others (pH 3.4-3.7) perhaps indicated the presence of a —NH3 group on the surface, which might mean more proteins were presented on the surface of solid substrate-grown cells[4-5, 19-20].
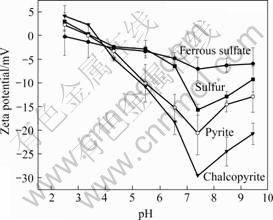
Fig.5 Zeta-potentials of A. manzaensis grown on different substrates
3.3 Hydrophobicity studies
The results of hydrophobicity of cells grown on different energy sources are shown in Fig.6. It is obvious that the cells cultured in solid substrate had higher hydrophobicity than the cells grown on ferrous sulfate, because the latter had a hydrophobicity of 1.2% in hexadecane organic phase, compared with the values of 6.5%, 16.17% and 17.75% of the cells grown on elemental sulfur, pyrite and chalcopyrite, respectively. Furthermore, the surface hydrophibicity of cells had subtle change with incubation time, which slightly increased in logarithmic-phase compared with the lag phase and decline phase. The results are in agreement with the studies of A. ferrooxidans and A.thiooxidans[4, 17]. Cell surface hydrophobicity is an important parameter in microbe-mineral interactions relevant to bioleaching. A stronger hydrophobicity of solid substrate-grown cells may help bacteria to adhere onto substrate, so as to facilitate the use of energy source.
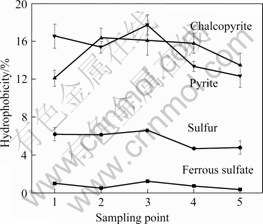
Fig.6 Hydrophobicity of A. manzaensis cells grown on different substrates
3.4 FT-IR studies
FT-IR spectra of A. manzaensis cells grown on ferrous ions, sulfur, pyrite and chalcopyrite are shown in Fig.7, and they present generally similar absorption features. The bands were assigned according to previous reports[5, 21]. Compared with the cells cultured in ferrous sulfate, the cells growing on solid substrate exhibit very strong absorption bands. A strong band at 3 300.6 cm-1 and a weaker band at 3 081.6 cm-1 are due to asymmetric and symmetric stretching of —NH2 group. The bands at 2 960, 2 928.3 and 2 870.5 cm-1 characterize asymmetric —CH3 stretching, asymmetric —CH2 stretching and symmetric —CH2 stretching, respectively. Very intense bands between 1 750 and 1 620 cm-1 are assigned to the —C=O group. The intense sharp band at 1 644.9 cm-1 indicates the presence of an amide group (amideⅠband). The bands at 1 457.9 and 1 377.6 cm-1 indicate the presence of —CH3 and —CH2 groups. The band at 1 261.5 cm-1 is due to —CH3 wagging modes. The band at 1 075.9 cm-1 is assigned to —CH3 wagging modes. These data indicate that proteins are contained on the surface of A.manzaensis. It is obvious that solid substrate-grown cells present stronger peaks than the cells grown on the soluble ferrous sulfate. Furthermore, among the three types of solid substrate-grown cells, the intensity of spectra of the sulfur-grown cells is weaker than that of pyrite- and chalcopyrite-grown cells. It can be concluded that the mineral substrate-grown cells have larger amounts of cell surface proteins than sulfur-grown cells. And the amount of protein on the surface of sulfur-grown cells is higher than that on the surface of ferrous ions-grown ones. The results of FT-IR spectra are in agreement with the results obtained from Zeta-potential and hydrophobicity tests. As protein plays an important role in cell attachment to substrate[5, 22], the larger amounts of proteins contained on substrate-grown cell surface indicates much hydrophobic characteristics. The different amounts of proteins may account for the difference of hydrophobicity and the surface charges under different conditions.

Fig.7 FT-IR spectra of A. manzaensis cells grown on different substrates: (a) Chalcopyrite; (b) Pyrite; (c) Sulfur; (d) Ferrous sulfate
4 Conclusions
1) The growth characteristics and surface properties of A. manzaensis YN25 cells were much dependent on the grown substrates.
2) The results of Zeta potentials showed that the solid substrate-grown cells had clearly higher isoelectric point (pH 3.4-3.7), among which the isoelectric point of chalcopyrite-grown cells was the highest of pH 3.7.
3) The results of hydrophobicity showed that the solid substrate-grown cells had higher hydrophobicity, and the results were in agreement with the result of Zeta potentials.
4) The results of FT-IR revealed that the larger amount of proteins was produced on A. manzaensis cell surface when cells were grown on sulfur and sulfide minerals.
References
[1] MANGOLD S, HARNEIT K, ROHWERDER T, CLAU G S, SANDW. Novel combination of atomic force microscopy and epifluorescence microscopy for visualization of leaching bacteria on pyrite [J]. Applied and Environmental Microbiology, 2008, 74(2): 410-415.
[2] LIU Jian-she YAN Ying WANG Hua-tai, WANG Xiu-mei. Progress in research on extracellular polymeric substance of Thiobacillus ferrooxidans [J]. Metal Mine, 2007, 378: 14-16.
[3] BLAKE R C, SHUTE E A, HOWARD G T. Solubilization of minerals by bacteria: Electrophoretic mobility of Thiobacillus ferrooxidans in the presence of iron, pyrite and sulfur [J]. Applied and Environmental Microbiology, 1994, 60(9): 3349-3357.
[4] DEVASIA P, NATARAJAN K A, SATHYANARAYANA D N, RAMANANDA RAO G. Surface chemistry of Thiobacillus ferrooxidans relevant to adhesion on mineral surfaces [J]. Applied and Environmental Microbiology, 1993, 59(12): 4051-4055.
[5] SHARMA P K, DAS A, HANUMANTHA RAO K, FORSSBERG K S E. Surface characterization of Acidithiobacillus ferrooxidans cells grown under different conditions [J]. Hydrometallurgy, 2003, 71: 285-292.
[6] KINZLER K, GEHRKE T, TELEGDI J, SAND W. Bioleaching—A result of interfacial processes caused by extracellular polymeric substances (EPS) [J]. Hydrometallurgy, 2003, 71: 83-88.
[7] SAND W, GEHRKE T. Extracellular polymeric substances mediate bioleaching/biocorrosion via interfacial processes involving iron(III) ions and acidophilic bacteria [J]. Research in Microbiology, 2006, 157(1): 49-56.
[8] KONISHI Y, TOKUSHIGE M, ASAI S, SUZUKI T. Copper recovery from chalcopyrite concentrate by acidophilic thermophile Acidianus brierleyi in batch and continuous-flow stirred tank reactors [J]. Hydrometallurgy, 2001, 59: 271-282.
[9] SHI Xian-ai, LI Cong-ying, LIN Hui, MENG Chun, GUO Yan-hao. Process characteristics of bioleaching Meizhou chalcopyrite by thermophilic Acidianus brierleyi [J]. The Chinese Journal of Process Engineering, 2005, 5(3): 333-336. (in Chinese)
[10] MENG Chun, SHI Xian-ai, LIN Hui, CHEN Jian-feng, GUO Yang-hao. UV induced mutations in Acidianus brierleyi growing in a continuous stirred tank reactor generated a strain with improved bioleaching capabilities [J]. Enzyme and Microbial Technology, 2007, 40(5): 1136-1140.
[11] MIKKELSEN D, KAPPLER U, WEBB R I, RASCH R, MCEWAN A G, SLY L I. Visualisation of pyrite leaching by selected thermophilic archaea: Nature of microorganism-ore interactions during bioleaching [J]. Hydrometallurgy, 2007, 88: 143-153.
[12] HARNEIT K, G?KSEL A, KOCK D, KLOCK J H, GEHRKE T, SAND W. Adhesion to metal sulfide surfaces by cells of Acidithiobacillus ferrooxidans, Acidithiobacillus thiooxidans and Leptospirillum ferrooxidans [J]. Hydrometallurgy, 2006, 83: 245-254.
[13] YOSHIDA N, NAKASATO M, OHMURA N, ANDO A, SAIKI H, ISHII M, IGARASHI Y. Acidianus manzaensis sp. nov., a novel thermoacidophilic archaeon growing autotrophically by the oxidation of H2 with the reduction of Fe3+ [J]. Current Microbiology, 2006, 53(5): 406-411.
[14] ROSENBERG M, GUTNICK D, ROSENBERG E. Adherence of bacteria to hydrocarbons: A simple method for measuring cell- surface hydrophobicity [J]. FEMS Microbiology Letters, 1980, 9(1): 29-33.
[15] SO C M, YOUNG L Y. Isolation and characterization of a sulfate-reducing bacterium that anaerobically degrades alkanes [J]. Applied and Environmental Microbiology, 1999, 65(7): 2969-2976.
[16] ROHWERDER T, GEHRKE T, KINZLER K, SAND W. Bioleaching review part A: Progress in bioleaching: Fundamentals and mechanisms of bacterial metal sulfide oxidation [J]. Applied Microbiology and Biotechnology, 2003, 63(3): 239-248.
[17] NATARAJAN K A, DAS A. Surface chemical studies on ‘Acidithiobacillus’ group of bacteria with reference to mineral flocculation [J]. International Journal of Mineral Processing, 2003, 72: 189-198.
[18] XIA Le-xia, LIU Xin-xing, ZENG Jia, YIN Chu, GAO Jian, LIU Jian-she, QIU Guan-zhou. Mechanism of enhanced bioleaching efficiency of Acidithiobacillus ferroxidans after adaptation with chalcopyrite [J]. Hydrometallurgy, 2008, 92: 95-101.
[19] BENDINGER B, RIJNAARTS H H M, ALTENDORF K, ZEHNDER A J B. Physicochemical cell surface and adhesive properties of coryneform bacteria related to the presence and chain length of mycolic acids [J]. Applied and Environmental Microbiology, 1993, 59(11): 3973-3977.
[20] RIJNAARTS H H M, NORDEB W, LYKLEMAB J, ZEHNDER A J B. The isoelectric point of bacteria as an indicator for the presence of cell surface polymers that inhibit adhesion [J]. Colloids and Surfaces B: Biointerfaces, 1995, 4(4): 191-197.
[21] SHARMA P K, HANUMANTHA RAO K. Surface characterization of bacterial cells relevant to the mineral industry [J]. Minerals and Metallurgical Processing, 2005, 22(1): 31-37.
[22] CLINTl J H, WICKS A C. Adhesion under water: Surface energy considerations [J]. International Journal of Adhesion and Adhesives, 2001, 21: 267-273.
Foundation item: Project(50674101) supported by the National Natural Science Foundation of China; Project(2004CB619201) supported by the National Basic Research Program of China; Project(50621063) supported by the Chinese Science Foundation for Distinguished Group
Corresponding author: XIA Jin-lan, Tel: +86-731-8836944; E-mail: jlxia@mail.csu.edu.cn
(Edited by YANG Bing)