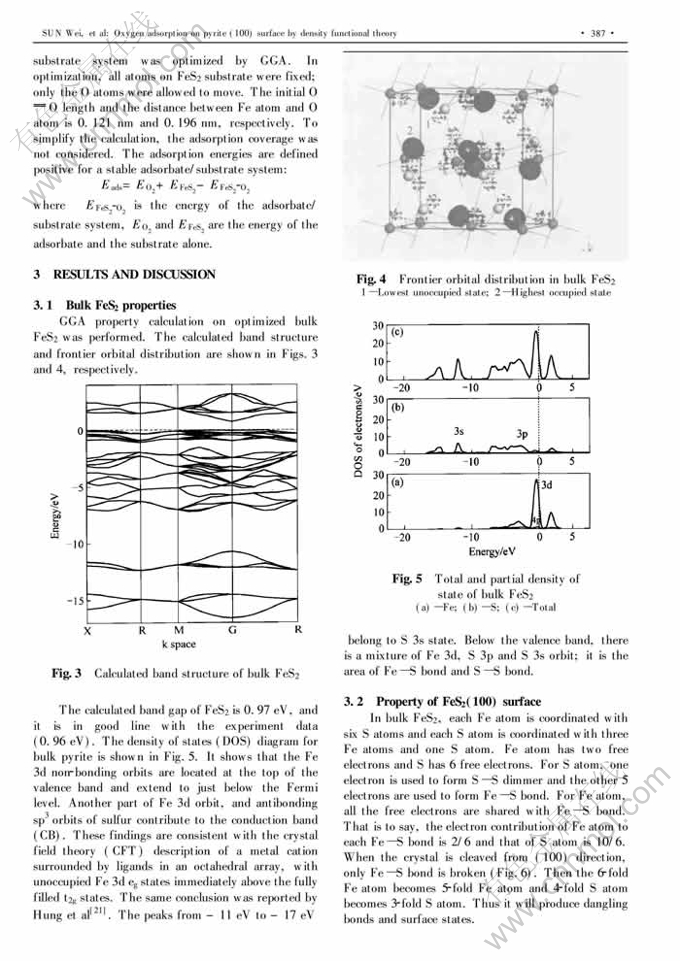
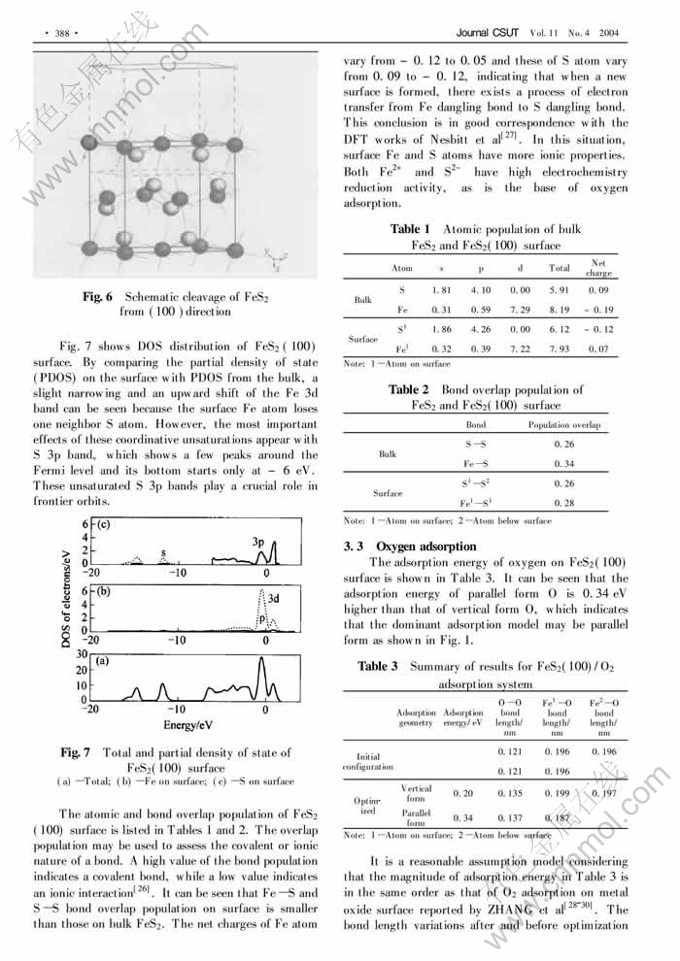
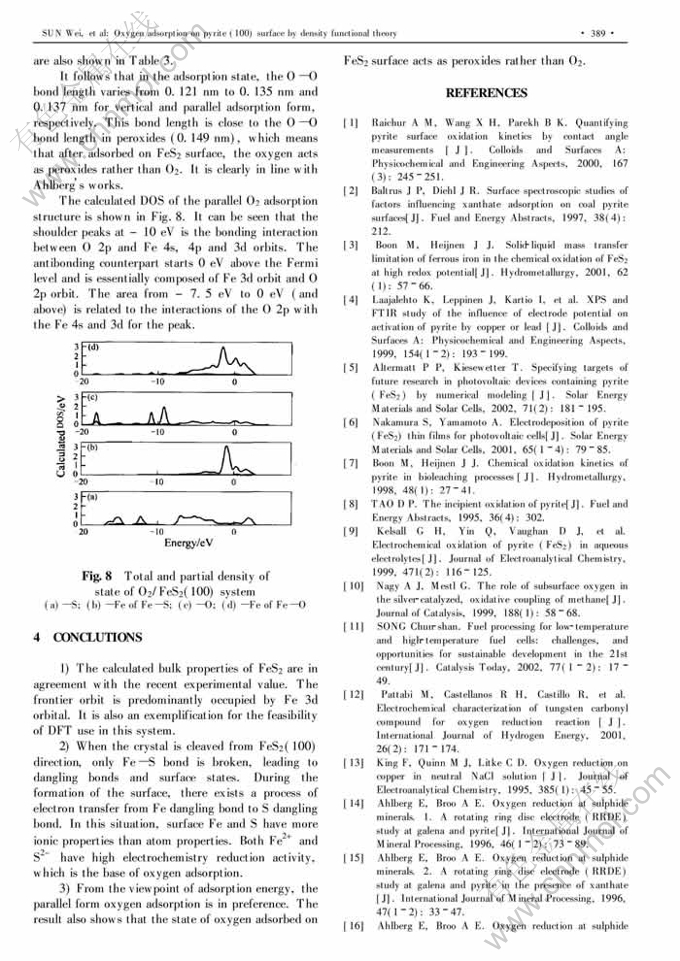
Oxygen adsorption on pyrite (100) surface by density functional theory①
来源期刊:中南大学学报(英文版)2004年第4期
论文作者:孙伟 胡岳华 邱冠周 覃文庆
文章页码:385 - 390
Key words:density functional theory; FeS2 (100) surface; surface relaxation; oxygen adsorption; sulfide flotation
Abstract: Pyrite (FeS2) bulk and (100) surface properties and the oxygen adsorption on the surface were studied by using density functional theory methods. The results show that in the formation of FeS2 (100) surface, there exists a process of electron transfer from Fe dangling bond to S dangling bond. In this situation, surface Fe and S atoms have more ionic properties. Both Fe2+and S2- have high electrochemistry reduction activity, which is the base for oxygen adsorption. From the viewpoint of adsorption energy, the parallel form oxygen adsorption is in preference. The result also shows that the state of oxygen absorbed on FeS2 surface acts as peroxides rather than O2.