黄铜矿精矿中等嗜热微生物浸出过程及其优化
来源期刊:中国有色金属学报2016年第5期
论文作者:王军 李旖旎 庄田 赵红波 朱珊 覃文庆 邱冠周
文章页码:1120 - 1129
关键词:黄铜矿;中等嗜热菌;生物浸出
Key words:chalcopyrite; moderate thermophilic bacteria; bioleaching
摘 要:采用3种中等嗜热微生物:喜温硫杆菌(Acidithiobacillus caldus,A.c)、嗜铁钩端螺旋菌(Leptospirillum ferriphilu,L.f)、嗜热氧化硫化杆菌(Sulfobacillus thermosulfidooxidans,S.t)对黄铜矿精矿进行浸出。探讨浸出过程中的微生物生长优化及搅拌反应器浸出条件优化。微生物最佳生长条件如下:生长温度为45 ℃、初始pH为1.5。驯化过的浸矿细菌的生长及浸出率明显高于未驯化的,驯化后浸出率在矿浆浓度为50 g/L时达到最大,为94.00%;当矿浆浓度达到100 g/L时,铜的浸出率稳定在80%左右。搅拌反应器的最优化浸出条件如下:搅拌速度350 r/min,充气强度500 mL/min。在此条件下,对黄铜矿精矿进行浸出,浸出时间为30 d时,最终铜离子浓度为17.36 g/L,铜的浸出率为85.60%。
Abstract: The bioleaching of chalcopyrite concentrate in the presence of three different moderate thermophilic bacteria such as Acidithiobacillus caldus (A.c), Leptospirillum ferriphilu (L.f) and Sulfobacillus thermosulfidooxidans (S.t), including the optimal conditions of microbial growth and the optimization of stirred reactor during the bioleaching process, was investigated. The results show that the best growth conditions of bacteria are as follows, temperature of 45 ℃ and initial pH of 1.5. The microbial growth of the domesticated bacteria and the bioleaching rate using them are significantly higher than those of the non-domesticated bacteria. The leaching rate using the domesticated strains reaches the maximum 94.00% with the pulp density of 50 g/L. The leaching rate of copper stabilizes at about 80% when the pulp density reaches 100 g/L. The optimal parameters of stirred reactors are as follows:stirring speed of 350 r/min and aeration intensity of 500 mL/min. The final concentration of copper ions is 17.36 g/L and the leaching rate of copper is 85.60% for the chalcopyrite bioleaching for 30 d under these conditions.
文章编号:1004-0609(2016)-05-1120-09
王 军1, 2,李旖旎1, 2,庄 田1, 2,赵红波1, 2,朱 珊1, 2,覃文庆1, 2,邱冠周1, 2
(1. 中南大学 资源加工与生物工程学院,长沙 410083;
2. 中南大学 生物冶金教育部重点实验室,长沙 410083)
摘 要:采用3种中等嗜热微生物:喜温硫杆菌(Acidithiobacillus caldus,A.c)、嗜铁钩端螺旋菌(Leptospirillum ferriphilu,L.f)、嗜热氧化硫化杆菌(Sulfobacillus thermosulfidooxidans,S.t)对黄铜矿精矿进行浸出。探讨浸出过程中的微生物生长优化及搅拌反应器浸出条件优化。微生物最佳生长条件如下:生长温度为45 ℃、初始pH为1.5。驯化过的浸矿细菌的生长及浸出率明显高于未驯化的,驯化后浸出率在矿浆浓度为50 g/L时达到最大,为94.00%;当矿浆浓度达到100 g/L时,铜的浸出率稳定在80%左右。搅拌反应器的最优化浸出条件如下:搅拌速度350 r/min,充气强度500 mL/min。在此条件下,对黄铜矿精矿进行浸出,浸出时间为30 d时,最终铜离子浓度为17.36 g/L,铜的浸出率为85.60%。
关键词:黄铜矿;中等嗜热菌;生物浸出
中图分类号:TD982 文献标志码:A
黄铜矿(CuFeS2)是一种重要的铜矿资源,在世界的硫化铜矿中约占70%[1-2]。从黄铜矿中提取铜的传统方法是火法冶金,但是焙烧会释放大量SO2等有害物质导致环境污染,相比之下采用生物冶金的方法提取铜是一种很好的手段[3],而如何有效地用生物浸出方法从黄铜矿中提取铜是人们所关注的[4-5]。然而,在浸出过程中,在黄铜矿表面会形成钝化膜,阻碍了浸出的进行[6]。
在生物浸出过程中,浸矿菌种的选择是影响浸矿效果的一个关键因素。在中温菌浸出中,会在矿物表面形成大量元素硫、中间硫化产物和黄钾铁矾等物质,从而导致矿物的表面钝化,使铜的浸出率较低[7-12];并且在堆浸过程中,由于浸矿菌种自身的新陈代谢关系,会造成温度的积累,使反应体系局部温度升高,因此常温菌很难存活。而极端高温菌没有细胞壁,耐金属离子浓度、矿浆浓度较低,在较高的剪切力下较难存活[13-14]。中等嗜热菌有坚固的细胞壁结构,在槽浸中能耐受较高的矿浆浓度和金属离子浓度,在堆浸中能耐受45 ℃的相对高温。使用中等嗜热菌浸出黄铜矿,不仅可以显著改善浸出的反应动力学,加快反应速率,缩短浸出周期,而且浸出过程中产生的硫化产物则会溶解,从而提高铜的浸出速率和浸出率[15-19]。另有研究表明[20-25],与单菌浸出相比用混合菌进行浸出极大地提高了浸出率。搅拌浸出具有能快速有效地进行反应、易于调控、金属回收率高等优点。但对搅拌速率、通气强度等有较高要求,一般适用于处理小批量矿石或精矿[26]。
温度、pH值、矿浆浓度、搅拌速率、充气强度等都对浸出有影响[27-29],本文作者通过对黄铜矿精矿浸出过程中的细菌生长条件(温度、初始pH值、对高矿浆浓度的驯化)及搅拌反应器条件(搅拌速率、充气强度)进行优化,达到提到浸出率的目的。
1 实验
1.1 矿样
实验中用的矿样来自中国云南省蒙自白牛厂矿区,样品进行了粉碎、磨矿并筛至粒径小于0.0074 mm。X射线衍射(XRD)和化学元素分析显示矿样由61.70%的CuFeS2、12.60%的闪锌矿(ZnS)、6.70%的氧化锡(SnO2)、3.90%的硫化铅(PbS)和3.70%的硫酸铅(PbSO4)组成,主要元素组成如下:Cu 21.46%、S 35.39%、Fe 23.90%、Zn 8.40%、Pb 5.02%(质量分数)。
1.2 微生物
本试验中采用的是3种中等嗜热菌:喜温硫杆菌(Acidithiobacillus caldus,A.c)、嗜铁钩端螺旋菌(Leptospirillum ferriphilu,L.f)、嗜热氧化硫化杆菌(Sulfobacillus thermosulfidooxidans,S.t)。这3种细菌是来自中国湖南长沙中南大学生物冶金菌种室。其中,A.c为硫氧化菌,L.f为亚铁氧化菌,S.t可同时氧化亚铁和硫。3株菌种使用改良9K培养基:((NH4)2SO4 3.0 g/L, Na2SO4 2.1 g/L, MgSO4·7H2O 0.5 g/L, K2HPO4 0.05 g/L, KCl 0.1 g/L 和Ca(NO3)2 0.01 g/L),另外添加20 g/L的硫酸亚铁(FeSO4)或5 g/L的单质硫(S0)为能源物质。3种中等嗜热菌混合培养(分别取1.5mL 菌浓度为1×108 mL-1的3种菌液,混合后接种到100 mL改良9K培养基中),形成共培养体系,随后试验都在此体系中进行。
1.3 菌种生长条件的优化
1.3.1 菌种最适生长温度的确定
将处于对数生长期的混合菌接种到改良9K培养基中,接种量为5%,设置温度梯度为37 ℃、41 ℃、45 ℃、49 ℃和53 ℃,将菌种置于此温度梯度的摇床中培养,转速为160 r/min。定时取样检测,采用血球计数板法,在光学显微镜(CX31RTSF)下观察微生物的数量,观察不同温度下细菌的生长情况。
1.3.2 菌种最适初始pH值的确定
用1:1(浓硫酸与水体积比)的硫酸(H2SO4)调节改良9K培养基的pH值,分别为1.0、1.1、1.2、1.3、1.4、1.5、1.6、1.7、1.8、1.9、2.0、2.1、2.2、2.3、2.4和2.5。将处于对数生长期的混合菌接种到此培养基中,接种量为5%(体积分数),置于45 ℃,160 r/min转速的摇床中培养3天后,定时取样检测,观察不同初始pH值对浸矿菌生长的影响。
1.3.3 菌种对高黄铜矿精矿矿浆浓度的驯化
驯化搅拌反应器(JHS-1)示意图如图1所示。将处于对数生长期的浸矿菌种接入到改良9K培养基中,接种量为10%,另加入20 g/L的FeSO4、5 g/L的S0和15 g/L的黄铜矿精矿。
保持搅拌槽夹套水温为45 ℃,在驯化初期采用1:1(浓硫酸与水体积比)的H2SO4使溶液pH保持在1.5。定期取样,用血球计数板法,在光学显微镜下观察微生物数量,当培养液中菌体浓度达到以1×108 mL-1时,过滤矿渣,收集浸出液,加入到改良9K培养基中,不加FeSO4和S0,按驯化的矿浆浓度梯度进行。在各矿浆浓度梯度下,用电感耦合等离子发射光谱仪(Inductively coupled plasma optical emission spectrometer,ICP-OES)检测浸出液中Cu2+浓度,当浸出液中铜离子浓度趋于稳定时,视为浸出实验结束。

图1 黄铜矿生物搅拌浸出反应器模型图
Fig. 1 Stirred tank bioreactor used for bioleaching of chalcopyrite concentrate
1.4 搅拌反应器浸出条件优化
黄铜矿精矿的生物浸出试验在10 L搅拌反应器(见图1)中进行,反应器外围中空可充水夹套恒温45 ℃,浸出初始pH值为1.5,接种量10%,矿浆浓度为100 g/L。生物浸出过程中用1:1的H2SO4或10 mol/L的NaOH维持pH值的稳定。
1.4.1 搅拌速率对生物浸出黄铜矿的影响
保持空气充入强度为500 mL/min(在充气管间接入空气过滤膜滤掉粉尘),而搅拌速度设为250、300、350和400 r/min。每隔3 d进行检测,用ICP-OES检测浸出液中铜离子浓度。
1.4.2 充气强度对生物浸出黄铜矿的影响
保持搅拌反应器搅拌速度为350 r/min,而充气强度设置梯度为300、400、500和600 mL/min。每隔3 d进行检测,用ICP-OES检测浸出液中铜离子浓度。
1.5 优化后的生物搅拌浸出试验
在如图1所示搅拌槽进行浸出试验。控制反应温度为45 ℃,搅拌速度为350 r/min,空气泵充气强度为500 mL/min,矿浆浓度为10%,接种量为10%,接种后的细菌浓度达到2.5×108 mL-1,浸出时间为30 d。
在浸出过程中,定期记录pH值和电位的变化,以及溶液中的铜的浓度、铁离子和细菌浓度。采用pH酸度计(BPP-922)测量pH值;以铂电极为工作电极,Ag/AgCl电极为参比电极的电位计(BPH-221),测量浸出液中的氧化还原电位;采用ICP-OES方法检测浸出液中铜、总铁离子浓度;采用硫酸铈滴定法测定浸出液内的亚铁离子浓度;浸出液中浸矿菌浓度通过血球计数板法在光学显微镜下观察微生物的数量。
2 结果与讨论
2.1 菌种生长条件优化
2.1.1 菌种最适生长温度的确定
在45 ℃的培养条件下,11 d后微生物进入生长稳定期,最高菌浓度达到4.5×108 mL-1(见图2);在41和49 ℃的培养条件下,微生物的最高菌浓度约为45 ℃条件下的一半;而在37和53 ℃培养条件下,细菌生长明显受到影响,最高菌浓度仅为4.0×107 mL-1左右,且其进入生长稳定期的时间较久,分别16和15 d。因而,得出混合菌最适生长温度为45 ℃。

图2 不同培养温度条件下中等嗜热菌群的最高菌浓度
Fig. 2 Cell densities in stable phase of moderate thermophiles under different temperatures
2.1.2 菌种最适初始pH值的确定
图3所示为不同初始pH下中等嗜热菌群的最高菌浓度。由图3可知,其绘制曲线类似于正态分布,当初始pH为1.5时,浸矿菌的生长情况最好,最高菌浓度达到4.5×108 mL-1;当初始pH增高或降低时,最高菌浓度均会降低;且当pH高于一定值时,细菌生长明显受到影响,如当初始pH值为2.2时,最高菌浓度不到1.0×108 mL-1,细菌生长受到抑制。因而,得出混合菌最适生长初始pH值为1.5,马鹏程等[30]也做过类似的研究,他们得出在初始pH值为1.4时,浸出体系中细菌的活性最好,矿物的氧化速率最快。
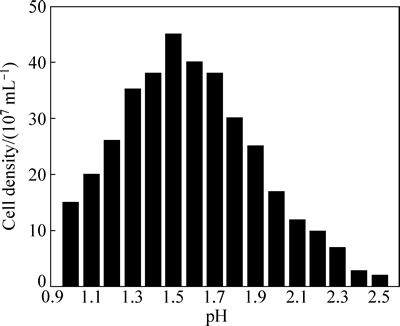
图3 不同初始pH条件下中等嗜热菌群的最高菌浓度
Fig. 3 Cell densities in stable phase of moderate thermophiles under different initial pH values
2.1.3 菌种对高黄铜矿精矿矿浆浓度的驯化
图4所示为中等嗜热菌群经数代驯化后的细菌浓度。浸矿菌第一代驯化矿浆浓度为15 g/L,第二代驯化矿浆浓度为30 g/L,第三代的为50 g/L,第四代的为75 g/L,第五代至第九代的均为100 g/L。由此可知,随着高矿浆浓度对浸矿菌的驯化,其活性逐渐能稳定在7.0×108 mL-1,表明其对高矿浆浓度的抗性增强,能在高黄铜矿矿浆浓度下达到较高的生长活性[31]。
图5所示为中等嗜热菌群在不同驯化代数下的浸出率。由图5可以看出,随着驯化代数的增加,浸矿菌对黄铜矿的浸出能力逐渐增强,对浸出液中高浓度铜离子的耐受能力也随之增强。铜的浸出率在矿浆浓度为50 g/L(即条柱上标注5.0%)达到最大,为94.00%;随着矿浆浓度的增加,浸出率有下降趋势,矿浆浓度达到100 g/L时,铜的浸出率稳定在80%左右。

图4 中等嗜热菌群经数代驯化后的细菌浓度
Fig. 4 Cell densities of moderate thermophiles during adaptive experiments

图5 中等嗜热菌群在不同驯化代数下的浸出率
Fig. 5 Copper extraction using moderate thermophiles domesticated for different times
可能是高矿浆浓度会造成反应体系中的剪切力过大,不利于微生物生长,从而影响到浸出率[32],当矿浆浓度超过50 g/L时,浸出率略微下降;且高矿浆浓度下,溶液中铜离子浓度过高会影响到微生物生长,从而也会影响到浸出率[33-34]。由此可知,对浸矿菌进行驯化能提高其对高矿浆浓度的抗性及对高铜离子浓度的耐受能力,从而能提高浸出效率。马鹏程等[35]就从多个矿坑、煤堆废水中富集中度嗜热浸矿菌,并在搅拌反应器中经过两年的长期驯化,获得了能耐受高矿浆浓度和具有抗砷性的浸矿混合菌。
2.2 搅拌反应器条件优化
2.2.1 搅拌速率对生物浸出黄铜矿的影响
由图6可知,当搅拌速度为350 r/min时,30 d内铜的浸出率最高,浸出铜离子浓度为17.36 g/L。当搅拌速度低于350 r/min时,搅拌速率不够,导致体系混合不均匀,溶氧不充分,会出现黄铜矿矿粉沉积,细菌没有与矿物充分作用,导致铜浸出率下降。当搅拌速度高于350 r/min时,铜浸出率降低,可能是搅拌速率过高,产生的剪切力过大,影响到细菌的生长繁殖,从而降低铜浸出速率[36]。由此可知,中度嗜热菌群搅拌浸出黄铜矿时最佳搅拌速度为350 r/min。
2.2.2 充气强度对生物浸出黄铜矿的影响
图7所示为反应器中不同充气强度对黄铜矿精矿生物浸出的影响。由图7可知,在充气强度为500 mL/min时,30 d内铜的浸出率最高,浸出铜离子浓度为17.36 g/L。当充气强度低于500 mL/min时,浸矿菌对黄铜矿的浸出速度较慢,尤其是在充气强度为300 mL/min时尤为明显,浸矿菌浸出速率特别低,可能是充气强度过低影响到了浸矿菌的生长和繁殖,从而影响到了浸出速率[37]。当充气强度为600 mL/min时,铜的浸出率反而降低,可能是充气强度过高,产生大量气泡,气泡破碎产生巨大剪切力影响到浸矿菌的生长繁殖,导致浸出率下降[36]。由此可知,中度嗜热菌群搅拌浸出黄铜矿时最佳充气强度为500 mL/min。
黄铜矿的生物浸出可分为两部分作用,化学作用及微生物作用,可表示为[38-39]:
CuFeS2+O2+4H+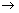 Cu2++Fe2++2S0+2H2O (化学作用)
Cu2++Fe2++2S0+2H2O (化学作用)
CuFeS2+4Fe3+ 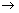 Cu2++5Fe2++2S0 (化学作用)
Cu2++5Fe2++2S0 (化学作用)
4Fe2++4H++O2 4Fe3++2H2O (微生物作用)
4Fe3++2H2O (微生物作用)
S0+3/2O2+H2O H2SO4 (微生物作用)
H2SO4 (微生物作用)
化学作用中主要是O2的氧化作用,因而充气强度会影响到浸出率。当微生物作用时,微生物充当催化剂的角色,即不断将亚铁离子氧化重新生成三价铁离子,并提供适宜的酸性环境;三价铁在酸性条件下是一种很有效的矿物氧化剂和浸出剂,能对黄铜矿具有较好的浸出作用;而在黄铜矿氧化分解过程中会释放亚铁离子,后者又是微生物代谢的能源物质,微生物又将其氧化为三价铁离子,如此构成一个循环,不断对黄铜矿浸出。O2在生物反应中会影响到细菌的生长代谢,CO2是此类微生物的碳源,用以合成自身生长所需的有机物,因此,充足的O2和CO2才能保证浸矿细菌的良好生长,从而保证浸矿效率。

图6 反应器中搅拌速度对生物浸出黄铜矿精矿的影响
Fig. 6 Effects of stirring rate on bioleaching of chalcopyrite in tank reactor
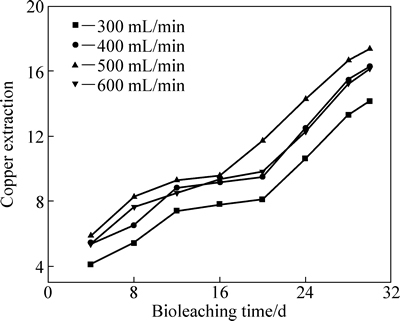
图7 反应器中不充气强度对黄铜矿精矿生物浸出的影响
Fig. 7 Effects of aeration intensities on the bioleaching of chalcopyrite in the tank reactor
2.3 优化后的生物搅拌浸出试验
图8所示为中等嗜热菌浸出过程中的生长曲线。由图8可知,驯化后的浸矿菌很好的适应了搅拌浸出黄铜矿的浸出环境,溶液中浸矿菌能迅速进入对数生长期,在14 d进入稳定期,菌浓度稳定在1.1×109 mL-1。而未驯化的浸矿菌生长受到抑制,这是由于其未经过驯化因而会受到高浓度矿浆的抑制,最高菌浓度仅为4.2×108 mL-1,远低于驯化后的浸矿菌浓度[31]。

图8 中等嗜热菌浸出过程中的生长曲线
Fig. 8 Growth curves of moderate thermophiles in tank reactor
图9所示为中等嗜热菌浸出黄铜矿精矿过程中铜离子浓度的变化。由图9可知,驯化后的浸矿菌的浸出率明显提高,30 d后,浸出液中铜离子浓度达到17.36 g/L,浸出率为85.60%;相比之下,未经过驯化的浸矿菌,在30 d后,浸出液中铜离子浓度仅为5 g/L,浸出率不到30%。这是由于驯化过后,浸矿菌能很好地适应高矿浆浓度,能很好地生长繁殖,从而得到较高的浸出率。

图9 中等嗜热菌浸出黄铜矿精矿过程中铜离子浓度的变化
Fig. 9 Copper extraction during bioleaching of chalcopyrite by moderate thermophiles in tank reactor
在中等嗜热菌群对黄铜矿精矿浸出过程,初始浸出速率很高,铜离子浓度迅速增加,而到第8 d后,进入一个平台期,直到第16 d后,又进入一个迅速浸出期,铜离子浓度再次迅速增加。很可能是在那段平台期,黄铜矿表面形成了一层钝化膜,阻碍了浸矿菌与黄铜矿的作用效果。许多学者都对此现象展开了研究[40-46]。一般认为,钝化膜形成主要是由于,浸矿微生物吸附到矿物表面后,会产生胞外多聚物,其能富集三价铁离子,从而氧化分解黄铜矿,但同时黄铜矿分解释放的单质硫也会附着在胞外多聚物上面,而随着反应的进行,溶液中的氧化还原电位和三价铁离子浓度逐步升高,会产生各种铁矾沉淀,其也会覆盖于矿物表面[8, 47-50]。
图10所示为中等嗜热菌浸出黄铜矿精矿过程中铁离子浓度的变化。由图10可知,由图10(a)可知,前8 d,三价铁浓度迅速增高,而在8~12 d略有下降,这主要是由于生成了黄钾铁矾,随后三价铁离子浓度急剧升高,马鹏程等[35]做的黄铜矿浸出试验中就对亚铁和三价铁的行为进行了详细的研究。
对比图10(a)和(b)可知,驯化过的浸矿菌对亚铁离子氧化效果明显高于未经过驯化的,驯化过的在第14 d就将亚铁离子氧化接近于0,而未经过驯化的直到28 d才接近于0。且比较其三价铁含量,驯化过的浸出后期三价铁含量明显高于未经过驯化的。综合其生长曲线可知,其铁离子浓度变化主要还是由于浸矿菌生长情况不同所造成的。

图10 中等嗜热菌浸出黄铜矿精矿过程中铁离子浓度的变化
Fig. 10 Variations of Ferrous and Ferric irons and total iron concentrations in chalcopyrite bioleaching experiment
3 结论
1) 通过对中等嗜热菌的最适生长条件(温度、初始pH值)进行优化。得出最佳培养条件为:生长温度为45 ℃、初始pH为1.5。
2) 使用黄铜矿矿浆浓度梯度对中等嗜热菌进行驯化,在矿浆浓度为50 g/L时,铜的浸出率达到94.00%;在矿浆浓度为100 g/L时,浸出率稳定在80%左右。相对于未经驯化的中等嗜热菌,驯化后的浸矿菌在最高细菌浓度和铜的浸出率方面均有较大提高。
3) 对搅拌反应器条件(搅拌速度、充气强度)的优化试验,结果表明在搅拌反应器中中等嗜热菌搅拌浸出黄铜矿精矿的最适搅拌速度为350 r/min,最适充气强度为500 mL/min。
4) 采用优化后的菌种生长条件及搅拌反应器条件,用中等嗜热菌黄铜矿精矿进行浸出,浸出时间为30 d,最终铜离子浓度为17.36 g/L,铜的浸出率为85.60%。
REFERENCES
[1] DUTRIZAC J E. The kinetics of dissolution of chalcopyrite in ferric ion media[J]. Metallurgical Transactions B, 1978, 9(3): 431-439.
[2] LI Y, KAWASHIMA N, LI J, CHANDRA A P, GERSON A R. A review of the structure, and fundamental mechanisms and kinetics of the leaching of chalcopyrite[J]. Advances in Colloid and Interface Science, 2013, 197: 1-32.
[3] 王晓冬, 段东平, 周 娥, 陈思明. 硫化铜矿强化浸出研究进展[J]. 中国有色冶金, 2014, 43(4): 38-41.
WANG Xiao-dong, DUAN Dong-ping, ZHOU E, CHEN Si-ming. Research progress of intensified leaching of copper sulfide ore[J]. China Nonferrous Metallurgy, 2014, 43(4): 38-41.
[4] BRIERLEY C L, BRIERLEY J A. Progress in bioleaching: part B: Applications of microbial processes by the minerals industries[J]. Applied Microbiology and Biotechnology, 2013, 97(17): 7543-7552.
[5] PANDA S, SANJAY K, SUKLA L B, PRADHAN N, SUBBAIAH T, MISHRA B K, PRASAD M S, RAY S K. Insights into heap bioleaching of low grade chalcopyrite ores: A pilot scale study[J]. Hydrometallurgy, 2012, 125: 157-165.
[6] TSHILOMBO A F. Mechanism and kinetics of chalcopyrite passivation and depassivation during ferric and microbial leaching[D]. New York: University of British Columbia, 2004.
[7] DUTRIZAC J. Elemental sulphur formation during the ferric sulphate leaching of chalcopyrite[J]. Canadian Metallurgical Quarterly, 1989, 28(4): 337-344.
[8] 曾伟民. 黄铜矿生物浸出过程中钝化膜的形成机制及其清除方法探讨[D]. 长沙: 中南大学, 2011.
ZENG Wei-min. The formation machanism of passivation layer and its elimination way during bioleaching of chalcopyrite[D]. Changsha: Central South University, 2011.
[9] KLAUBER C, PARKER A, van BRONSWIJK W, WATLING H. Sulphur speciation of leached chalcopyrite surfaces as determined by X-ray photoelectron spectroscopy[J]. International Journal of Mineral Processing, 2001, 62(1): 65-94.
[10] ZHAO Hong-bo, WANG Jun, QIN Wen-qing, HU Ming-hao, ZHU Shan, QIU Guang-zhou. Electrochemical dissolution process of chalcopyrite in the presence of mesophilic microorganisms[J]. Minerals Engineering, 2015, 71: 159-169.
[11] YANG Yi, LIU Wei-hua, CHEN Miao. A copper and iron K-edge XANES study on chalcopyrite leached by mesophiles and moderate thermophiles[J]. Minerals Engineering, 2013, 48: 31-35.
[12] XIA Le-xian, LU Tang, XIA Jin-lan, CHU Yin, CAI Li-yuan, ZHAO Xiao-juan, NIE Zhen-yuan, LIU Jian-she, QIU Guan-zhou. Relationships among bioleaching performance, additional elemental sulfur, microbial population dynamics and its energy metabolism in bioleaching of chalcopyrite[J]. Transactions of Nonferrous Metals Society of China, 2012, 22(1): 192-198.
[13] KONISHI Y, ASAI S, TOKUSHIGE M, SUZUKI T. Kinetics of the bioleaching of chalcopyrite concentrate by acidophilic thermophile Acidianus brierleyi[J]. Biotechnology Progress, 1999, 15(4): 681-688.
[14] 刘 凯, 刁梦雪, 杨 宇, 覃文庆, 吴学玲. 混合高温菌浸出黄铜矿及浸出过程中微生物群落的演替[J]. 中国有色金属学报, 2015, 20(2): 346-353.
LIU Kai, DIAO Meng-xue, YANG Yu, QIN Wen-qing, WU Xue-ling. Bioleaching of chalcopyrite concentrate using mixed thermophilic culture and succession of microbial community during leaching process[J]. The Chinese Journal of Nonferrous Metals, 2015, 20(2): 346-353.
[15] RODRIGUEZ Y, BALLESTER A, BLAZQUEZ M, GONZALEZ F, MUNOZ J. New information on the chalcopyrite bioleaching mechanism at low and high temperature[J]. Hydrometallurgy, 2003, 71(1): 47-56.
[16] SAND W, ROHDE K, SOBOTKE B, ZENNECK C. Evaluation of Leptospirillum ferrooxidans for leaching[J]. Applied and Environmental Microbiology, 1992, 58(1): 85-92.
[17] BRIERLEY J A. Thermophilic iron-oxidizing bacteria found in copper leaching dumps[J]. Applied and Environmental Microbiology, 1978, 36(3): 523-525.
[18] BRIERLEY J, LOCKWOOD S. The occurrence of thermophilic iron-oxidizing bacteria in a copper leaching system[J]. FEMS Microbiology Letters, 1977, 2(3): 163-165.
[19] BRIERLEY J A, NORRIS P R, KELLY D P, LE ROUX N W. Characteristics of a moderately thermophilic and acidophilic iron-oxidizing Thiobacillus[J]. European journal of applied microbiology and biotechnology, 1978, 5(4): 291-299.
[20] ZHOU Hong-bo, LIU Xi, FU Bo, QIU Guan-zhou, HUO Qiang, ZENG Wei-min, LIU Jian-she, CHEN Xin-hua. Isolation and characterization of Acidithiobacillus caldus from several typical environments in China[J]. Journal of Central South University of Technology, 2007, 14(2): 163-169.
[21] FU Bo, ZHOU Hong-bo, ZHANG Ru-bing, QIU Guan-zhou. Bioleaching of chalcopyrite by pure and mixed cultures of Acidithiobacillus spp. and Leptospirillum ferriphilum[J]. International Biodeterioration & Biodegradation, 2008, 62(2): 109-115.
[22] KINNUNEN P H M, PUHAKKA J A. Characterization of iron-and sulphide mineral-oxidizing moderately thermophilic acidophilic bacteria from an Indonesian auto-heating copper mine waste heap and a deep South African gold mine[J]. Journal of Industrial Microbiology and Biotechnology, 2004, 31(9): 409-414.
[23] WU Chang-bin, ZENG Wei-min, ZHOU Hong-bo, FU Bo, HUANG Ju-fang, QIU Guan-zhou, WANG Dian-zuo. Bioleaching of chalcopyrite by mixed culture of moderately thermophilic microorganisms[J]. Journal of Central South University of Technology, 2007, 14(4): 474-478.
[24] FENG Shou-shuai, YANG Hai-lin, XIN Yu, GAO Kai, YANG Ji-wei, LIU Ting, ZHANG Ling, WANG Wu. A novel and highly efficient system for chalcopyrite bioleaching by mixed strains of Acidithiobacillus[J]. Bioresource Technology, 2013, 129: 456-462.
[25] WEI Zhu, XIA Jin-lan, PENG An-an, NIE Zhen-yuan, QIU Guan-zhou. Characterization of apparent sulfur oxidation activity of thermophilic archaea in bioleaching of chalcopyrite[J]. Transactions of Nonferrous Metals Society of China, 2013, 23(8): 2383-2388.
[26] 刘漫博, 孙 琦, 李国平, 李路路, 范 燕. 生物冶金技术应用与发展现状[J]. 甘肃冶金, 2012, 34(5): 1-3.
LIU Man-bo, SUN Qi, LI Guo-ping, LI Lu-lu, FAN Yan. Application and development of bioleaching technology[J]. Gansu Metallurgy, 2012, 34(5): 1-3.
[27] QIN Wen-qing, YANG Cong-ren, LAI Shao-shi, WANG Jun, LIU Kai, ZHANG Bo. Bioleaching of chalcopyrite by moderately thermophilic microorganisms[J]. Bioresource Technology, 2013, 129: 200-208.
[28] WANG Yu-guang, SU Li-jun, ZENG Wei-min, WAN Li-li, CHEN Zhu, ZHANG Li-juan, QIU Guan-zhou, CHEN Xin-hua, ZHOU Hong-bo. Effect of pulp density on planktonic and attached community dynamics during bioleaching of chalcopyrite by a moderately thermophilic microbial culture under uncontrolled conditions[J]. Minerals Engineering, 2014, 61: 66-72.
[29] KAKSONEN A H, MORRIS C, REA S, LI J, WYLIE J, USHER K M, GINIGE M P, CHENG K Y, HILARIO F, du PLESSIS C A. Biohydrometallurgical iron oxidation and precipitation: Part I—Effect of pH on process performance[J]. Hydrometallurgy, 2014, 147/148: 255-263.
[30] 马鹏程, 杨洪英, 王路平, 杨培根, 刘 慧. pH 对黄铜矿细菌浸铜的影响[J]. 有色金属(冶炼部分), 2015(3): 1-4.
MA Peng-cheng, YANG Hong-ying, WANG Lu-ping, YANG Pei-gen, LIU Hui. Effect of pH value on bioleaching of chalcopyrite[J]. Nonferrous Metals (Extractive Metallurgy), 2015(3): 1-4.
[31] 吴俊子, 曾伟民, 王玉光, 仉丽娟, 万利利, 周洪波. 搅拌槽反应器中中度嗜热浸矿菌预处理含砷金矿[J]. 过程工程学报, 2013, 13(3): 494-499.
WU Jun-zi, ZENG Wei-min, WANG Yu-guang, ZHANG Li-juan, WAN Li-li, ZHOU Hong-bo. Pretreatment of refractory gold ore by a moderate thermophilic consortium in stirred tank reactor[J]. The Chinese Journal of Process Engineering, 2013, 13(3): 494-499.
[32] GERICKE M, GOVENDER Y, PINCHES A. Tank bioleaching of low-grade chalcopyrite concentrates using redox control[J]. Hydrometallurgy, 2010, 104(3): 414-419.
[33] 张广积, 方兆珩. 驯化氧化亚铁硫杆菌从镍黄铁矿中浸出镍[J]. 过程工程学报, 2001, 1(3): 285-288.
ZHANG Guang-ji, FANG Zhao-heng. Bioleaching of nicopyrite with adapted thiobacillus ferrooxidans[J]. The Chinese Journal of Process Engineering, 2001, 1(3): 285-288.
[34] MOUSAVI S, YAGHMAEI S, VOSSOUGHI M, JAFARI A, HOSEINI S. Comparison of bioleaching ability of two native mesophilic and thermophilic bacteria on copper recovery from chalcopyrite concentrate in an airlift bioreactor[J]. Hydrometallurgy, 2005, 80(1): 139-144.
[35] 马鹏程, 杨洪英, 佟琳琳, 韩战旗, 宋 言. 黄铜矿生物浸出过程中Fe(Ⅱ)和Fe(Ⅲ)的行为[J]. 中国有色金属学报, 2013, 23(6): 1694-1700.
MA Peng-cheng, YANG Hong-ying, TONG Lin-lin, HAN Zhan-qi, SONG Yan. Behaviour of Fe(Ⅱ) and Fe(Ⅲ) in chalcopyrite bioleaching process[J]. The Chinese Journal of Nonferrous Metals, 2013, 23(6): 1694-1700.
[36] 张卫民, 谷士飞, 孙占学. 催化条件下低品位原生硫化铜矿的搅拌细菌浸出[J]. 金属矿山, 2009(4): 40-42.
ZHANG Wei-min, GU Shi-fei, SUN Zhan-xue. Agitation bioleaching of low-grade primary copper sulfide ore under caterlysis conditions[J]. Mine, 2009(4): 40-42.
[37] TORMA A, WALDEN C, DUNCAN D, BRANION R. The effect of carbon dioxide and particle surface area on the microbiological leaching of a zinc sulfide concentrate[J]. Biotechnology and Bioengineering, 1972, 14(5): 777-786.
[38] ZHAO Hong-bo, WANG Jun, YANG Cong-ren, HU Ming-hao, GAN Xiao-wen, TAO Lang, QIN Wen-qin, QIU Guan-zhou. Effect of redox potential on bioleaching of chalcopyrite by moderately thermophilic bacteria: An emphasis on solution compositions[J]. Hydrometallurgy, 2015, 151: 141-150.
[39] LIANG Chang-li, XIA Jin-lan, YANG Yi, NIE Zhen-yuan, ZHAO Xiao-juan, ZHENG Lei, MA Chen-yan, ZHAO Yi-dong. Characterization of the thermo-reduction process of chalcopyrite at 65°C by cyclic voltammetry and XANES spectroscopy[J]. Hydrometallurgy, 2011, 107(1/2): 13-21.
[40] CRUNDWELL F. How do bacteria interact with minerals?[J]. Hydrometallurgy, 2003, 71(1/2): 75-81.
[41] SCHIPPERS A, SAND W. Bacterial leaching of metal sulfides proceeds by two indirect mechanisms via thiosulfate or via polysulfides and sulfur[J]. Applied and Environmental Microbiology, 1999, 65(1): 319-321.
[42] GEHRKE T, TELEGDI J, THIERRY D, SAND W. Importance of extracellular polymeric substances from Thiobacillus ferrooxidans for bioleaching[J]. Applied and Environmental Microbiology, 1998, 64(7): 2743-2747.
[43] GOMEZ C, ROMAN E, BLAZQUEZ M, BALLESTER A. SEM and AES studies of chalcopyrite bioleaching in the presence of catalytic ions[J]. Minerals Engineering, 1997, 10(8): 825-835.
[44] 傅开彬, 董发勤, 谌 书, 王维清, 徐龙华, 王 振. 黄铜矿生物浸出钝化及调控研究进展[J]. 武汉理工大学学报, 2013, 35(6): 128-133.
FU Kai-bin, DONG Fa-qin, CHEN Shu, WANG Wei-qing, XU Long-hua, WANG Zhen. Review on passivation and elimination way during bioleaching of chalcopyrite[J]. Journal of WuHan University of Technology, 2013, 35(6): 128-133.
[45] 梁长利, 夏金兰, 杨 益, 聂珍媛, 邱冠周. 黄铜矿生物浸出过程的硫形态转化研究进展[J]. 中国有色金属学报, 2012, 22(1): 265-273.
LIANG Chang-li, XIA Jin-lan, YANG Yi, NIE Zhen-yuan, QIU Guan-zhou. Progress in sulfur speciation transformation during chalcopyrite bioleaching[J]. The Chinese Journal of Nonferrous Metals, 2012, 22(1): 265-273.
[46] ZHAO Hong-bo, WANG Jun, QIN Wen-qing, ZHENG Xi-hua, TAO Lang, GAN Xiao-wen, QIU Guan-zhou. Surface species of chalcopyrite during bioleaching by moderately thermophilic bacteria[J]. Transactions of Nonferrous Metals Society of China, 2015, 25(8): 2725-2733.
[47] SAND W, GEHRKE T. Extracellular polymeric substances mediate bioleaching/biocorrosion via interfacial processes involving iron (III) ions and acidophilic bacteria[J]. Research in Microbiology, 2006, 157(1): 49-56.
[48] ZENG Wei-min, QIU Guan-zhou, ZHOU Hong-bo, LIU Xue-duan, CHEN Miao, CHAO Wei-liang, ZHANG Cheng-gui, PENG Juan-hua. Characterization of extracellular polymeric substances extracted during the bioleaching of chalcopyrite concentrate[J]. Hydrometallurgy, 2010, 100(3): 177-180.
[49] 莫晓兰, 林 海, 温建康, 徐承焱. 脉石矿物对细菌浸出黄铜矿的影响研究[J]. 稀有金属, 2013, 37(3): 437-445.
MO Xiao-lan, LIN Hai, WEN Jian-kang, XU Cheng-yan. Effect of gangue minerals on chalcopyrite bioleaching[J]. Chinese Journal of Rare Metals, 2013, 37(3): 437-445.
[50] YU Run-lan, JING Liu, AN Chen, ZHONG Dai-li, QIAN Li, QIN Wen-qing, QIU Guan-zhou, GU Guo-hua. Interaction mechanism of Cu2+, Fe3+ ions and extracellular polymeric substances during bioleaching chalcopyrite by Acidithiobacillus ferrooxidans ATCC2370[J]. Transactions of Nonferrous Metals Society of China, 2013, 23(1): 231-236.
WANG Jun1, 2, LI Yi-ni1, 2, ZHUANG Tian1, 2, ZHAO Hong-bo1, 2,
ZHU Shan1, 2, QIN Wen-qing1, 2, QIU Guan-zhou1, 2
(1. School of Minerals Processing and Bioengineering, Central South University, Changsha 410083, China;
2. Key Laboratory of Biometallurgy, Ministry of Education, Central South University, Changsha 410083, China)
Abstract: The bioleaching of chalcopyrite concentrate in the presence of three different moderate thermophilic bacteria such as Acidithiobacillus caldus (A.c), Leptospirillum ferriphilu (L.f) and Sulfobacillus thermosulfidooxidans (S.t), including the optimal conditions of microbial growth and the optimization of stirred reactor during the bioleaching process, was investigated. The results show that the best growth conditions of bacteria are as follows, temperature of 45 ℃ and initial pH of 1.5. The microbial growth of the domesticated bacteria and the bioleaching rate using them are significantly higher than those of the non-domesticated bacteria. The leaching rate using the domesticated strains reaches the maximum 94.00% with the pulp density of 50 g/L. The leaching rate of copper stabilizes at about 80% when the pulp density reaches 100 g/L. The optimal parameters of stirred reactors are as follows: stirring speed of 350 r/min and aeration intensity of 500 mL/min. The final concentration of copper ions is 17.36 g/L and the leaching rate of copper is 85.60% for the chalcopyrite bioleaching for 30 d under these conditions.
Key words: chalcopyrite; moderate thermophilic bacteria; bioleaching
Foundation item: Projects (51374248, 51320105006) supported by the National Natural Science Foundation of China; Project (20120162120010) supported by the Research Foundation for the Doctoral Program of Higher Education, China; Project (NCET 13 0595) supported by the Program for New Century Excellent Talents in University, China
Received date: 2015-08-25; Accepted date: 2015-12-08
Corresponding author: WANG Jun; Tel: +86-731-88876557; E-mail: wjwq2000@126.com
(编辑 龙怀中)
基金项目:国家自然科学基金资助项目(51374248,51320105006);教育部博士点新教师基金资助项目(20120162120010);教育部新世纪人才计划项目(NCET 13 0595)
收稿日期:2015-08-25;修订日期:2015-12-08
通信作者:王 军,副教授,博士;电话:0731-88876557;E mail:wjwq2000@126.com

