


Trans. Nonferrous Met. Soc. China 22(2012) 858-864
Synthesis and electrochemical properties of Zn2SnO4 and Zn2Sn0.8Ti0.2O4/C
YUAN Wan-song, TIAN Yan-wen, LIU Li-juan, LI Jian-zhong
School of Materials and Metallurgy, Northeastern University, Shenyang 110819, China
Received 18 April 2011; accepted 15 June 2011
Abstract: Compound Zn2Sn0.8Ti0.2O4 was synthesized by a hydrothermal method in which SnCl4·5H2O, TiCl4, ZnCl2 and N2H4·H2O were used as reactants. The composite Zn2Sn0.8Ti0.2O4/C was then prepared through a carbothermic reduction process using the as-prepared Zn2Sn0.8Ti0.2O4 and glucose as reactants. The structure, morphology and electrochemical properties of the as-prepared products were investigated by XRD, XPS, TEM and electrochemical measurements. In addition, electrochemical Li insertion/extraction in composite Zn2Sn0.8Ti0.2O4/C were examined by ex situ XRD and SEM. The first discharge capacity of Zn2SnO4 is about 1670.8 mA·h/g, with a capacity retain of 342.7 mA·h/g in the 40th cycle at a constant current density of 100 mA/g in the voltage range of 0.05-3.0 V. Comparing with the Zn2SnO4, some improved electrochemical properties are obtained for Zn2Sn0.8Ti0.2O4, Zn2SnO4/C and Zn2Sn0.8Ti0.2O4/C. The composite Zn2Sn0.8Ti0.2O4/C shows the best electrochemical properties, and its first discharge capacity is about 1530.0 mA·h/g, with a capacity retain of 479.1 mA·h/g the 100th cycle.
Key words: Zn2SnO4; Zn2Sn0.8Ti0.2O4/C; titanium substitution; lithium ion batteries; anode materials
1 Introduction
The increasing demand for higher energy density and power density of batteries in electronic devices has attracted investigators to develop new materials for lithium-ion batteries. In commercial lithium-ion batteries, graphite is used as an anode material. However, the theoretical capacity of graphite is only 372 mA·h/g [1-3]. Compound Zn2SnO4 has sparked more interest recently because it can deliver more capacity. When lithium ion inserts into or extracts from it, the following reactions can take place [4-7]:
4Li++Zn2SnO4+4e=Sn+2Li2O+2ZnO (1)
8Li++Zn2SnO4+8e=2Zn+Sn+4Li2O (2)
xLi++Sn+xe=LixSn (0≤ x ≤ 4.4) (3)
yLi++Zn+ye=LiyZn (y ≤ 1) (4)
When lithium ions insert into Zn2SnO4 particles, they will form metallic Sn and Zn as well as Li-Sn and Li-Zn alloys. It can be seen that about 14.4 lithium ions will react with Zn2SnO4, Sn and Zn. Based on the above reactions, the theoretical values of irreversible and reversible capacity were calculated to be about 1231 and 547 mA·h/g, respectively. Nevertheless, the most critical problem for Zn2SnO4 to be used as anode is the severe volume expansion and contraction during the alloying/de-alloying process of the lithium ion with Sn and Zn. Furthermore, metallic Sn and Zn will conglomerate. The large volume change during alloying and de-alloying reactions generates severe stress which results in cracks and crumbling of the particles. This deterioration causes loss of electronic contact between the bulk particles, which gives poor cycle life. To overcome the problem, some improved measures must be taken.
In this study, a hydrothermal method is used to prepare Zn2Sn0.8Ti0.2O4, and then the as-prepared Zn2Sn0.8Ti0.2O4 reacts with glucose to produce composite Zn2Sn0.8Ti0.2O4/C. To our best knowledge, the electrochemical features of composite Zn2Sn0.8Ti0.2O4/C are rarely reported.
2 Experimental
2.1 Synthesis procedure
The pure Zn2SnO4 was synthesized by hydrothermal reaction at 180 ℃ according to the method previously reported by YUAN et al [7]. The Zn2Sn0.8Ti0.2O4 was also synthesized by hydrothermal reaction under the same conditions as synthesis of Zn2SnO4. In this process, analytical grade reagents SnCl4·5H2O, TiCl4, ZnCl2 and N2H4·H2O were used as reactants. The molar ratio of Zn to Sn to Ti to N2H4·H2O was 2:0.8:0.2:8. Zinc chloride, tin chloride, and titanium chloride were dissolved into distilled water to form transparent solution under magnetic stirring. N2H4·H2O was then added dropwise into the mixture to form white slurry. After stirring for 30 min, the final mixture was transferred into a Teflon-lines stainless steel autoclave with a filling capacity of 80%. The autoclave was maintained at 180 ℃ for 24 h, and cooled naturally to room temperature. The precipitate was filtered and washed with ethanol and deionized water several times. Finally, the product was dried at 80 ℃ for 12 h.
After Zn2SnO4 and Zn2Sn0.8Ti0.2O4 were obtained, the composites Zn2SnO4/C and Zn2Sn0.8Ti0.2O4/C were then prepared through a carbothermic reduction process, respectively. The as-prepared Zn2SnO4 and Zn2Sn0.8Ti0.2O4 were separately mixed with glucose (C6H12O6) together at a mass ratio of 85:15. This mixture was put into distilled water under magnetic stirring, and then dried at 80 ℃. At last, the dried mixtures were separately calcined at 600 ℃ for 2 h under Ar atmosphere to obtain composite Zn2SnO4/C and Zn2Sn0.8Ti0.2O4/C.
2.2 Analysis of active materials
The prepared samples were identified and characterized on a X-ray powder diffractometer (Rigaku Co. Ltd). X-ray profiles were measured between 10° and 80° (2θ angle) with a monochromatic Cu Kα radiation source. Transmission electron microscopy (TEM, FEI Tecnai 12) was also employed to characterize the as-prepared samples. X-ray photoelectron spectra (XPS) were measured at room temperature on an ESCALAB 250. During XPS analysis, the Al Ka X-ray beam was adopted as the excitation source, and the power was set to be 150 W. Vacuum pressure of the instrument chamber was 1?10-7 Pa read on the panel. In addition, SEM was also used to observe the change of electrodes in morphology and microstructure before and after given cycles.
2.3 Electrochemical test
In order to evaluate the electrochemical characteristics, the working electrode was prepared by pressing a mixture of the active material, conducting carbon black, and binder (PVDF) in a mass ratio of 70:15:15. Li foil was used as the counter and reference electrodes. The electrolyte was 1 mol/L LiPF6 in a mixture of ethylene carbonate (EC), diethyl carbonate (DMC), ethylene methyl carbonate (EMC) with a volume ratio of 1:1:1. The cells were assembled in an argon-filled dry box. Charge-discharge tests were performed in the voltage range of 0.05-3.0 V at the current density of 100 mA/g.
3 Results and discussion
3.1 Structure characterization of materials
Zn2SnO4 belongs to the inverse spinel structure where the tetrahedral voids are occupied by the Zn atoms and the octahedral voids are occupied by equal number of Zn and Sn atoms [8]. X-ray diffraction patterns of the Zn2SnO4, Zn2Sn0.8Ti0.2O4, Zn2SnO4/C and Zn2Sn0.8Ti0.2O4/C are shown in Fig. 1. It is clearly seen that all diffraction peaks of the four samples are agree well with the reverse spinel structure of Zn2SnO4 (JCPDS, Card No. 24—1470), suggesting that these materials prepared under our conditions are well-crystallized and partial substitution of Ti for Sn in the Zn2SnO4 will not change the crystal structure. Figure 1 also reveals that some diffraction peaks of the samples with Ti-doping undergo a slight shift toward higher degrees. For clear observation, the peak position of (440) planes of Zn2SnO4 and Zn2Sn0.8Ti0.2O4 are magnified and shown in Fig. 2. In addition, the diffraction peak intensities of Zn2SnO4 and Zn2Sn0.8Ti0.2O4/C are stronger than that of the Zn2SnO4 because they were heat-treated at 600 ℃.

Fig. 1 XRD patterns of Zn2SnO4, Zn2Sn0.8Ti0.2O4 and Zn2SnO4/C and Zn2Sn0.8Ti0.2O4/C
The lattice parameters for Zn2SnO4 and Zn2Sn0.8Ti0.2O4 samples were calculated by least square fitting as 8.6550 ? and 8.6452 ? based on the XRD peaks, respectively, which were in accordance with the standard XRD data (JCPDS, Card No. 24—1470). The chemical valences of Ti, Sn and Zn in the Zn2Sn0.8Ti0.2O4 are +4, +4 and +2, respectively. The ionic radii of Ti4+, Sn4+ and Zn2+ are 0.61 ?, 0.71 ? and 0.74 ?, respectively. Because the ionic radius of Ti4+ is smaller than that of Sn, which is substituted for the lattice constant of Zn2Sn0.8Ti0.2O4 is smaller than that of Zn2SnO4.
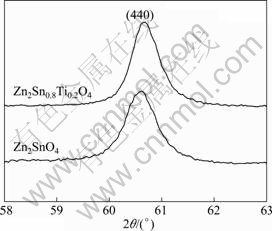
Fig. 2 Enlarged (440) peaks of Zn2SnO4 and Zn2Sn0.8Ti0.2O4 in Fig. 1
It is well known that the X-ray photoelectron spectrum (XPS) is an effective tool for the study of material surface and to investigate the chemical state of elements in compound. Figure 3 presents XPS of the Zn2SnO4 and Zn2Sn0.8Ti0.2O4. The peaks in the Zn2SnO4, Zn 2p (1021.79 eV), Sn 3d (486.0 eV) and O1s (530.3 eV) are detected. The peaks of the Zn 2p and Sn 3d spectra for the Ti-doping material almost have no inspective change on the binding energy, compared with Zn2SnO4, indicating that the Zn and Sn ion environments in the structure were not changed after doping Ti. Meanwhile, the XPS spectrum of Ti is shown in Fig. 3(d). The XPS spectrum of Ti 2p3/2 shows the characteristic peaks at 458.62 eV, which is similar to the binding energies of Ti4+ in TiO2 [9].
3.2 Morphology of materials
Figures 4 (a) and (b) display TEM images of the as-prepared Zn2SnO4 and composite Zn2Sn0.8Ti0.2O4/C samples, respectively. It can be seen that Zn2SnO4 particles have irregular shapes and consist of a number of small crystallites with a diameter between 20 and 30 nm. Composite Zn2Sn0.8Ti0.2O4/C particles also exhibit irregular shapes but the particle size is a bit larger.
3.3 Electrochemical performance and changes of electrode structure and morphology
The charge-discharge curves of Zn2SnO4 and composite Zn2Sn0.8Ti0.2O4/C are shown in Fig. 5. For Zn2SnO4, its first discharge and charge capacities are about 1670.8 and 689.5 mA·h/g, respectively. The irreversible capacity in the first discharge is up to 981.3 mA·h/g, which is different from the subsequent discharge. Based on Eq. (1) to Eq. (4), the theoretical capacity derived from the maximum uptake of 14.4 Li/Zn2SnO4 is about 1231 mA·h/g, which is smaller than that of the initial discharge. This is probably caused by the formation of a solid electrolyte interphase (SEI) film [10]. The large irreversible discharge capacity after the first cycle is probably due to severe side reaction with the electrolyte to form Li2O and SEI film, especially for the nano-Zn2SnO4 particles with the smaller size and larger surface area. There is almost no capacity above the 1.0 V in the first discharge curve and a wide platform at 1.0-0.5 V can be observed. However, from the second cycle afterward, all the potential plateaus are very similar and display more inclined shape. An obvious result is that the plateau in the first cycle disappears in the subsequent cycles owing to the change of the active electrode material after the first cycle. To further confirm the electrode change in structure, the XRD patterns of the Zn2SnO4 material and the Zn2SnO4 specimen taken from the electrode at the charged state and discharged state after one cycle are presented in Fig. 6. Three phases, inversed spinel Zn2SnO4, Zn and Sn, are detected at the charged state. It is found that most Li-Sn and Li-Zn alloys can be reversibly decomposed. In the case of the discharged state, the diffraction peaks of inversed spinel Zn2SnO4 disappear, but Zn and Sn exist. This proves that in the discharge process the Zn2SnO4 particles are reduced to Zn and Sn. Meanwhile, amorphous Li2O as well as Li-Sn and Li-Zn alloys are also generated. There is an obvious tendency for capacity to reduce. For example, the charge capacities in the 2nd, 10th and 40th cycle are 694.9, 648.3 and 342.7 mA·h/g, respectively. Its capacity retention is only 49.3% of the first charge capacity after 40 cycles.

Fig. 3 XPS spectra of pure phase Zn2SnO4 and Zn2Sn0.8Ti0.2O4 (a), Zn (b), Sn (c) and Ti (d)

Fig. 4 TEM images of Zn2SnO4 (a) and composite Zn2Sn0.8Ti0.2O4/C (b)

Fig. 5 Charge-discharge curves of product: (a) Zn2SnO4; (b) Composite Zn2Sn0.8Ti0.2O4/C

Fig. 6 XRD patterns of Zn2SnO4 electrode before and after the first charge-discharge: (a) Initial state; (b) At discharge state; (c) At charge state
The first discharge and charge capacities of the composite Zn2Sn0.8Ti0.2O4/C electrode are 1530.0 and 752.3 mA·h/g, respectively. The composite Zn2Sn0.8Ti0.2O4/C electrode shows an initial columbic efficiency of approximately 49.2%, which is higher than that of Zn2SnO4 (41.8%). From the second cycle, the columbic efficiency increases to more than 90%. It is considered that this should behave in a similar electrochemically manner to Zn2SnO4, although the products are slightly different.
XRD is also applied to analyzing the composite Zn2Sn0.8Ti0.2O4/C electrode change in structure. The XRD pattern of the composite Zn2Sn0.8Ti0.2O4/C specimen taken from the electrode at the charged state and discharged state after one cycle is presented in Fig. 7. Four phases, inversed spinel Zn2SnO4, Zn, Sn and TiO2 are detected at the charged state. It is found that most Li-Sn and Li-Zn alloys can be reversibly decomposed. In the case of the discharged state, the diffraction peaks of inversed spinel Zn2SnO4 disappear, but Zn and Sn exist. This proves that in the discharge process the composite Zn2Sn0.8Ti0.2O4/C particles are also reduced to Zn and Sn as Zn2SnO4. Meanwhile, TiO2 and LiTiO2 are also detected.

Fig. 7 XRD patterns of composite Zn2Sn0.8Ti0.2O4/C electrode after the first charge-discharge: (a) At discharge state; (b) At charge state
For titania, the lithium insertion / extraction to TiO2 proceeds according to the following reversible reaction: TiO2+xLi++xe=LixTiO2. The maximum x for the reversible reaction of normal crystalline anatase TiO2 is 0.5, which corresponds to a capacity of 168 mA·h/g [11]. During the insertion process, the crystal structure of LixTiO2 changes from tetragonal Li0.05TiO2 to orthorhombic Li0.5TiO2, but it can maintain the structure stability during the charge-discharge process [12]. Moreover, the active TiO2 and inactive LiTiO2 are useful for enhancing the mechanical properties of the matrix by alleviating the volume expansion/contraction of active (Sn, Zn) nanocrystallites, which occurs during cycling within a nanostructure composite [13-15].
The cycling behaviors of Zn2SnO4, Zn2Sn0.8Ti0.2O4, Zn2SnO4/C and Zn2Sn0.8Ti0.2O4/C electrodes are shown in Fig. 8. Although the Zn2SnO4 electrode delivers very high initial discharge capacity, its capacity retention is quite poor. The Zn2Sn0.8Ti0.2O4, Zn2SnO4/C and Zn2Sn0.8Ti0.2O4/C electrodes deliver an initial discharge capacity of 1548.4, 1512.7 and 1530.0 mA·h/g, respectively, which are just a bit lower than that of Zn2SnO4 electrode. However, they show much better capacity retention than Zn2SnO4 electrode. After 40 cycles, the charge capacity of Zn2Sn0.8Ti0.2O4, composites Zn2SnO4/C and Zn2Sn0.8Ti0.2O4/C remain at 413.4, 500.1 and 674.5 mA·h/g, respectively. The capacity retentions of Zn2Sn0.8Ti0.2O4, Zn2SnO4/C and Zn2Sn0.8Ti0.2O4/C are 72.5%, 51.9% and 89.6%, respectively. Obviously, the capacity retention of the Zn2Sn0.8Ti0.2O4/C is excellent compared with Zn2Sn0.8Ti0.2O4 and Zn2SnO4/C electrodes. It suggests that a synergistic effect may exist between Ti element in this composite and carbon matrix during cycling. The Zn2Sn0.8Ti0.2O4/C electrode shows a very stable capacity of 479.1 mA·h/g over 100 cycles, which is even much higher than the theoretical capacity of graphite. From the first to the 100th cycle, the charge capacity for Zn2Sn0.8Ti0.2O4/C decreases from 752.3 to 497.1 mA·h/g, with a little average capacity fading of 2.5 mA·h/g per cycle, which shows the best capacity retention of this material advanced so far [4-7]. The improvement of electrochemical behavior should be attributed to the unique composite structure consisting of active Zn, Sn and TiO2, and inactive LiTiO2 in the carbon matrix. Firstly, the amorphous carbon in composite can dissociate the metallic Sn and Zn particles and improve a high electrical conductivity for composites. Secondly, the TiO2 itself is electroactive material for lithium storage and contributes to overall capacity of electrodes. Thirdly, the active TiO2 and inactive LiTiO2 can not only enhance mechanical properties of the matrix to accommodate the stress generated during cycling, but also dissociate the metal Sn and Zn particles.

Fig. 8 Cycling performance of products
In order to shed the light on the enhanced electrochemical performance of material via doping, the morphological changes of the composite electrodes after different cycles were examined by SEM (Fig. 9). After 20 cycles, severe agglomeration appears in the Zn2SnO4 electrode, while there is no significant change in microstructure of Zn2Sn0.8Ti0.2O4/C electrode. This indicates that the amorphous carbon, the active TiO2 and inactive LiTiO2 can dissociate the metallic Sn and Zn particles, and the degree of Sn and Zn particles conglomerate can be decreased. The present results agree well with the above analysis.
4 Conclusions
1) The composite Zn2Sn0.8Ti0.2O4/C was synthesized by a hydrothermal method and subsequent carbothermic reduction process. The structure, morphology and electrochemical properties of Zn2Sn0.8Ti0.2O4/C were investigated by XRD, XPS, TEM and electrochemical measurements.
2) The XRD and XPS results show that Ti is indeed incorporated into the Zn2SnO4 crystal structure.
3) The composite Zn2Sn0.8Ti0.2O4/C shows a better electrochemical performance than most previously reported Zn2SnO4 samples, with a much higher capacity of 479.1 mA·h/g after 100 cycles.
4) The larger capacity and better cyclability of the composite Zn2Sn0.8Ti0.2O4/C electrode are attributed to composite structure consisting of active Zn, Sn and TiO2, and inactive LiTiO2 in the carbon matrix during the cycling process.
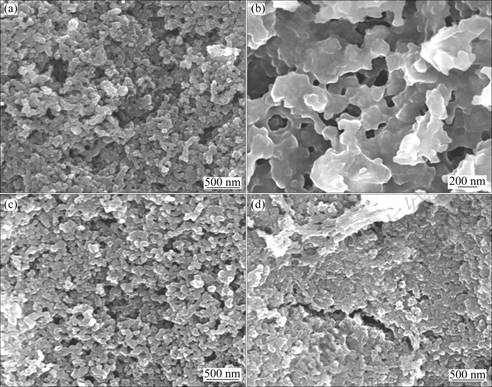
Fig. 9 SEM images of Zn2SnO4 electrode (a, b) and Zn2Sn0.8Ti0.2O4/C electrode (c, d): (a), (c) Before cycle; (b), (d) After 20 cycles
References
[1] WU Y P, JIANG C, WU C, HOLZE R. Anode materials for lithium ion batteries by oxidative treatment of common natural graphite [J]. Solid State Ionics, 2003, 156(3-4): 283-290.
[2] BUKKAYD D, NABAIS C, MERCIER C, SCHNEIDER R, WILLMANN P. Electrochemical lithium insertion in graphite containing dispersed tin–antimony alloys [J]. Energy Convers Manage, 2008, 49: 2447-2454.
[3] HUGGINS R A. Alternative materials for negative electrodes in lithium systems [J]. Solid State Ionics, 2002, 152-153: 61-68.
[4] RONG A, GAO X P, LI G R, YAN T Y, ZHU H Y, QU J Q, SONG D Y. Hydrothermal synthesis of Zn2SnO4 as anode materials for Li-ion battery [J]. J Phys Chem B, 2006, 110: 14754-14760.
[5] ZHU X J, GENG L M, ZHANG F Q, LIU Y X, CHENG L B. Synthesis and performance of Zn2SnO4 as anode materials for lithium ion batteries by hydrothermal method [J]. J Power Sources, 2009, 189(1): 828-831.
[6] HOU X G, CHENG Q, BAI Y, ZHANG W F. Preparation and electrochemical characterization of Zn2SnO4 as anode materials for lithium ion batteries [J]. Solid State Ionics, 2010, 181(3-4): 631-634.
[7] YUAN W S, TIAN Y W, LIU G Q. Synthesis and electrochemical properties of pure phase Zn2SnO4 and composite Zn2SnO4/C[J]. J Alloys Compd, 2010, 506: 683-687.
[8] FU Z L, YANG H K, MOON B K, CHOI B C, JEONG J H. Hydrothermal synthesis and optical characteristics of Eu3+ in Zn2SnO4nanocrystals [J]. Current Applied Physics, 2009, 9: 1360-1364.
[9] HAO Y J, LAI Q Y, LIU D Q, XU Z U, JI X Y. Synthesis by citric acid sol–gel method and electrochemical properties of Li4Ti5O12anode material for lithium-ion battery [J]. Mater Chem Phys, 2005, 94(2-3): 382-387.
[10] XIAO T, TANG Y W, JIA Z Y, FENG S L. Synthesis of SnO2/Mg2SnO4nanoparticles and their electrochemical performance for use in Li-ion battery electrodes [J]. Electrochim Acta, 2009, 54(8): 2396-2401.
[11] LINDSTROM H, SODERGREN S, SOLBRAND A, RENSMO H, HJELM J, HAGFELDT A, LINDQUIST S E. Li+ ion insertion in TiO2 (anatase): 1. Chronoamperometry on CVD films and nanoporous films [J]. J Phys Chem B, 1997, 101(39): 7710-7716.
[12] ZHAO Z W, GUO Z P, WEXLER D, MA Z F, XU X, LIU H L. Titania nanotube supported tin anodes for lithium intercalation [J]. Electrochem Commun, 2007, 9(4): 697-702.
[13] PARK C M, CHANG W S, JUNG H, KIM J H, SOHN H J. Nanostructured Sn/TiO2/C composite as a high-performance anode for Li-ion batteries [J]. Electrochem Commun, 2009, 11: 2165-2168.
[14] JEONG G J, KIM Y U, SOHN H J, KANG T. Particulate-reinforced Al-based composite material for anode in lithium secondary batteries [J]. J Power Sources, 2001, 101(2): 201-205.
[15] MAO O, TURNER R L, COURTNEY I A, FREDERICKSEN B D, BUCKETT M I, KRAUSE L J, DAHN J R. Electrochem active/inactive nanocomposites as anodes for Li-ion batteries [J]. Solid-State Lett, 1999, 2(1): 3-5.
Zn2SnO4和Zn2Sn0.8Ti0.2O4/C的制备和电化学性能
袁万颂,田彦文,刘丽娟,李建中
东北大学 材料与冶金学院,沈阳 110819
摘 要:以SnCl4·5H2O、TiCl4、ZnCl2和N2H4·H2O为原料,采用水热法制备Zn2Sn0.8Ti0.2O4纳米粉体。在此基础上,以葡萄糖和水热合成的Zn2Sn0.8Ti0.2O4为原料,以碳热还原法制备Zn2Sn0.8Ti0.2O4/C复合材料。利用XRD、XPS、TEM、恒电流充放电等方法分别研究Zn2SnO4和Zn2Sn0.8Ti0.2O4/C复合材料的结构、形貌和电化学性能。同时用非原位XRD、XPS 和SEM分析Zn2Sn0.8Ti0.2O4/C复合材料电极在充放电过程中的结构和形貌变化。合成的纯Zn2SnO4的首次放电容量为1670.8 mA·h/g,循环40次后放电容量迅速衰减为342.7 mA·h/g。而Zn2Sn0.8Ti0.2O4/C复合材料的首次放电容量为1530.0 mA·h/g,循环100次后容量还保持为479.1 mA·h/g,与纯Zn2SnO4、Zn2Sn0.8Ti0.2O4和Zn2SnO4/C相比,电化学性能有较大的提高。
关键词:Zn2SnO4;Zn2Sn0.8Ti0.2O4/C;钛掺杂;锂离子电池;负极材料
(Edited by LI Xiang-qun)
Foundation item: Project (51004028) supported by the National Natural Science Foundation of China
Corresponding author: TIAN Yan-wen; Tel: +86-24-83684943; E-mail: yuanwansong2010@163.com
DOI: 10.1016/S1003-6326(11)61257-0