
Reaction mechanism between oxide film on surface of Al-Li alloy and CsF-AlF3 flux
XUE Song-bai(薛松柏), ZHANG Ling(张 玲), HAN Zong-jie(韩宗杰), HUANG Xiang(黄 翔)
College of Materials Science and Technology, Nanjing University of Aeronautics and Astronautics,Nanjing 210016, China
Received 13 March 2007; accepted 12 September 2007
Abstract: The composition of the oxide film on the surface of Al-Li alloy was measured after accelerated oxidation at 500 and 540 ℃, and the reaction mechanism between CsF-AlF3 flux and the oxide film on the surface of Al-Li alloy was also discussed. The results show that the oxide film on the surface of Al-Li alloy is mostly composed of Li2CO3 and amorphous Al2O3. The brazing technology for Al-Li alloy is accomplished using the improved CsF-AlF3 flux. The improved flux CsF-AlF3 can effectively remove the oxide film by the way of reacting and/or dissolving the oxide film, of which CsF compound plays an important role in the course of removing the oxide film. The key step is the generation of HF, which induces and accelerates the reaction of removing oxide film. The generation of H2O also accelerates the reaction.
Key words: aluminum-lithium alloy; oxide film; flux; fluoride
1 Introduction
Li is the lightest metal element, and the density of Al alloys is reduced by about 3% and the elastic modulus is increased by about 6% with each 1% (mass fraction) addition of Li to the alloys[1-3]. Al-Li alloys are very attractive for aeronautic and astronautic applications. But the applications of Al-Li alloys are limited because the welding process is interfered by the oxide film on the surface of Al-Li alloy composed of complicated oxide compounds such as Li2CO3, Li2O, Al2O3 and Li compounds such as LiH, LiOH, LiAlO2[4-5], and the joint strength is much lower than the strength of the base alloys[6]. Although the tensile strength of weld is up to 345 MPa by friction stir welding, it is still much lower than that of the base alloy[7]. With the help of furnace brazing, the tensile strength of weld achieves 385 MPa (the average value), which is the highest value so far, and the strength index reaches 0.88. It is likely to find wider applications in aeronautics and astronautics industry. It is considered that brazing is one of the best jointing methods, but the key of obtaining good brazing joint is choosing the appropriate flux and brazing temperature.
In this study, the mechanism of flux of KF-AlF3 (the melting point is about 558 ℃) and CsF-AlF3 (the melting point is about 508 ℃) respectively reacting with oxide film on the surface of Al-Li alloy was researched. Owing to the temperature of solution treatment of Al-Li alloy is 520 ℃, the brazing temperature was set at (520±20) ℃. Preparatory experimental results indicated that the brazing technology for the Al-Li alloy was accomplished using CsF-AlF3 flux which can effectively remove the oxide films at 500-540 ℃, so the mechanism of CsF-AlF3 flux reacting with the oxide film on the surface of Al-Li alloy was analyzed mainly.
2 Experimental
2.1 Materials and equipments
The experimental base metals were melted by routine Al alloy melting furnace. The compositions were close to those of 1460 Al-Li alloy, and the chemical compositions of the Al-Li alloy are listed in Table 1. The thickness of the hot-rolled plate was 2.36 mm. After solution treatment at 520 ℃ for 10 min, water quenching, 4% pre-drawing and aging at 160 ℃ for 24 h, the tensile strength of the plate was measured to be 440 MPa and the elongation of the plate was 11%.
Table 1 Chemical compositions of Al-Li alloy (mass fraction, %)
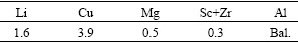
The fluxes used were the KF-AlF3 flux and the improved CsF-AlF3 flux.
Before the experiments, the plates of Al-Li alloy were cleaned by acetone, and treated by 15% NaOH solution at 50-60 ℃ for 15 s, then the plates were flushed by clean water. The 1?3 HNO3 solution was used to deal with the plates for about 10 s. After the flush of clean water again, ethanol was used to clean them. When the plates were dried naturally, they were ready for the experiments. The heating equipment was the SX-4-10 cabinet-type resistance furnace.
2.2 Method
When the temperature of resistance furnace was elevated to 500 ℃, the specimen was placed into the resistance furnace for 6 h. After cooling in the air, the oxide film on the surface of Al-Li alloy was scraped. The procedure was repeated for several times in order to collect enough oxide, and the oxide film was named sample 1. Similarly the collected oxide at 540 ℃ was named sample 2. The CsF-AlF3 flux was mixed with water into paste, then it was coated on the surface of the plates homogeneously with the thickness of about 0.1 mm. When the temperature of resistance furnace was elevated to 500 ℃, the coated plate was placed into the resistance furnace for 15 min. After cooling in the air, the residue was scraped, which was named sample 3. Similarly the residue obtained at 540 ℃ was named sample 4.
3 Results
The CsF-AlF3 flux and the four samples were analyzed by X-ray diffractometry (Model X'TRA). The results are shown in Fig.1.
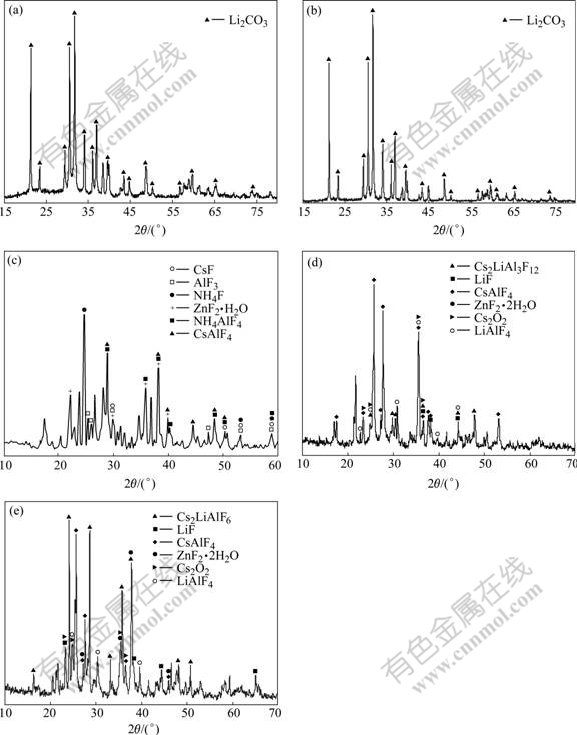
Fig.1 XRD patterns of samples: (a) Oxide film at 500 ℃; (b) Oxide film at 540 ℃; (c) CsF-AlF3 flux; (d) Residue of flux reacted with oxide film at 500 ℃; (e) Residue of flux reacted with oxide film at 540 ℃
Figs.1(a) and (b) show that the diffraction peaks of oxide film on the surface of Al-Li alloy after accelerated oxidation at 500 and 540 ℃ are basically the same, and the main reaction product is Li2CO3. Fig.1(c) shows that the compositions of CsF-AlF3 flux are CsAlF4, AlF3, CsF, NH4F, NH4AlF4 and ZnF2·2H2O. Comparing Fig.1(c) with Figs.1(d) and (e), the flux sufficiently reacts with the oxide film on the surface of Al-Li alloy at 500 and 540 ℃. The main residues are CsAlF4, LiF, Cs2LiAl3F12, LiAlF4, Cs2O2, ZnF2·2H2O at 500 ℃ and Cs2LiAlF6, CsAlF4, LiAlF4, LiF, Cs2O2, ZnF2·2H2O at 540 ℃. In a word, the reaction between the CsF-AlF3 flux and the oxide film on the surface of Al-Li alloy is sufficient at 500-540 ℃.
However the active temperature of KF-AlF3 flux is over 572 ℃[8]. This temperature is above the burning temperature of the base metal (the burning temperature of Al-Li alloys is 560-600 ℃), so KF-AlF3 flux isn’t appropriate to brazing the Al-Li alloys, and the mechanism of the KF-AlF3 flux reacting with the oxide film on the surface of the Al-Li alloy isn’t further researched.
4 Discussion
4.1 Analysis of oxide film
The results of X-ray diffraction of the oxide film on the surface of Al-Li alloy after accelerated oxidation at 500℃ and 540 ℃ were indexed by computer and handwork. They show that the observed diffraction peaks match with the monoclinic phase Li2CO3 and no diffraction peaks of Al2O3 appear. This is because that Al2O3 in the oxide film on the surface of Al-Li alloy is amorphous at room temperature. Al2O3 is still amorphous at 500-540 ℃ because there is no enough energy for Al2O3 to transform from amorphous to crystal [9-11]. The results are the same as the existing form of Al2O3 in oxide film of Al-Li-Cu-Zr alloy researched by PRASAD et al[12].
At room temperature, all compounds at the interface of air/oxide film contain Li. With the elevation of temperature, the thickness of oxide film increases gradually. The amount of Li compound on the surface increases obviously after heating for long time. Since the affinity of Li to oxygen is big and Li is enriched on the surface during heating, Li reacts with O2 and gaseous H2O in the air to form the Li compounds on the original amorphous Al2O3 oxide film such as Li2CO3, LiOH, LiAlO2 and Li2O[4-5]. With prolonging the heating time, the Li compound is mainly Li2CO3 at 500-540 ℃ because Li2O and LiOH reacts with CO2 in the air to form Li2CO3[13-14]. The chemical reactions of Li2O and LiOH with CO2 are the following:
Li2O+CO2=Li2CO3 (1)
2LiOH+CO2=Li2CO3+H2O↑ (2)
4.2 Comparison between flux and residue of flux
The diffraction peaks of the residue of flux reacted with the oxide film on the surface of alloy at 500 and 540 ℃ shown in Figs.1(d) and (e) are far from those of the CsF-AlF3 flux shown in Fig.1(c) and those of oxide film shown in Figs.1(a) and (b). This shows that the CsF-AlF3 flux reacts with the oxide film on the surface of Al-Li alloy sufficiently after heating at 500 and 540 ℃. The phases of flux, sample 3 and 4 are listed in Table 2.
Table 2 Phases of flux, sample 3 and sample 4
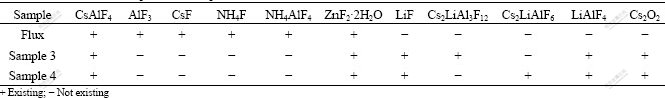
The residues are basically the same at 500 and 540 ℃, as listed in Table 2. The difference is that the residue contains Cs2LiAl3F12 at 500 ℃ and contains Cs2LiAlF6 at 540 ℃. The content of Cs2LiAlF6 at 540 ℃ is the largest, which shows that the higher the temperature is, the more sufficient the reactions are (500-540 ℃).
4.3 Mechanism of flux reacting with oxide film
The role of the flux is divided into three parts in brazing: matrix, stripper and interfacial agent. The earlier researchers thought[8] that the oxide film of Al alloys is removed by dissolving into chloride flux containing fluoride. However, WEST and SULLY[8] considered that Al2O3 can hardly dissolve into the molten flux, only can be peeled off mechanically and suspend in the molten flux. For the Nocolok flux, the behavior of removing the oxide film is the process of reacting and/or dissolving the oxide film, and the stronger ability of removing the oxide film is related to the existence of 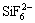 and Zn2+. The CsF-AlF3 flux is improved based on the KF-AlF3 Nocolok flux, therefore the mechanism of the CsF-AlF3 flux removing the oxide film is similar to that of the KF-AlF3 flux, namely removing the oxide film in the way of reaction and/or dissolution.
and Zn2+. The CsF-AlF3 flux is improved based on the KF-AlF3 Nocolok flux, therefore the mechanism of the CsF-AlF3 flux removing the oxide film is similar to that of the KF-AlF3 flux, namely removing the oxide film in the way of reaction and/or dissolution.
The CsF-AlF3 flux coated on the surface of Al-Li alloy plate can lead to the following reaction at 500-540 ℃[15].
CsAlFe4![]() CsF+AlF3 (3)
CsF+AlF3 (3)
CsF and AlF3 are not found in the flux residue, however CsAlF4 is present, which shows that the reaction above is reversible. In the brazing process, CsAlF4 is decomposed to CsF and AlF3 in the heating process, while CsF reacts with AlF3 to form CsAlF4 in the cooling process.
4.3.1 Inducing and accelerating role of HF to reaction of removing oxide film
Minor NH4AlF and NH4F exist in the flux. HF can be produced from NH4AlF and NH4F due to their instability at elevated temperatures, and HF can induce and accelerate the following reactions[5,15]:
NH4AlF4=NH4F+AlF3 (4)
NH4F=HF↑+NH3↑ (5)
CsAlF4+H2O=CsF+Al2O3+2HF↑ (6)
2AlF3+3H2O=Al2O3+6HF3 (7)
Li2O+2HF=2LiF+H2O↑ (8)
LiOH+HF=LiF+H2O↑ (9)
2LiAlO2+2HF=2LiF+Al3O2+H2O↑ (10)
Li2CO3+2HF=2LiF+H2O↑+CO2↑ (11)
The reactions show that CsF-AlF3 flux removing the oxide film on the surface of Al-Li alloy is related to the formation of HF. HF reacts with Li compounds in the oxide film on the surface of Al-Li alloy, such as Li2CO3, LiOH, LiAlO2, Li2O to form LiF, indicating that the CsF-AlF3 flux can remove the Li compounds in the oxide film on the surface of Al-Li alloy effectively.
The reactions (6) and (7) indicate that gaseous H2O plays an important role in the course of removing the oxide film[8,15]. The existing or formed water promotes the formation of HF, therefore the process of removing oxide film is accelerated.
4.3.2 Deduction of main residue of reaction of removing oxide film
Figs.1(d) and (e) show that there exists Cs2O2. Because of its instable chemical property, Cs2O can be further oxidized by O2 in the air to form Cs2O2 at 500- 540 ℃. Cs2O is the product of CsF reacting with Li2O and Al2O3:
8CsF+Li2O+Al2O3=2LiF+2AlF3+4Cs2O (12)
2Cs2O+O2=2Cs2O2 (13)
LiF, the product of flux reacting with the oxide film on the surface of Al-Li alloy further reacts with CsF and AlF3, which are the products of flux at the temperature of brazing. The residue is different at different temperatures, which shows that the extent of reaction is different. X-ray diffraction analysis indicates that the residue contains Cs2LiAl3F12 at 500 ℃ and Cs2LiAlF6 at 540 ℃. The following reactions can be deduced[12,15]:
2CsF+3AlF3+LiF=Cs2LiAl3F12 (14)
2CsF+AlF3+LiF=2Cs2LiAlF6 (15)
Fig.1(d) shows that the content of Cs2LiAl3F12 is little at 500 ℃, while Fig.1(e) shows that the content of Cs2LiAlF6 is large at 540 ℃. The reactions of CsF-AlF3 flux and the oxide film are sufficient at 540 ℃. The fact implies that the higher the temperature is, the more sufficient the reactions are (at 500-540 ℃).
LiAlF4 exists in the residue of flux, because LiF reacts with AlF3 in the flux to form LiAlF4 at 500-540 ℃[17]:
LiF+AlF3=LiAlF4 (16)
It is well known that Al2O3 cannot be dissolved by acid but can be dissolved by alkali. The aluminum ingot is obtained by electrolyzing Al2O3 which is dissolved in molten Na3AlF6. In a similar way, minor amount of Al2O3 on the surface of Al-Li alloy is dissolved in CsAlF4, consequently a compact and homogeneous slag layer is formed on the surface of alloy for protecting. In the process of CsAlF4 reacting with the oxide film on the surface of Al-Li alloy, CsF-AlF3 flux begins to melt at 450-480 ℃ and the formed compound is still stable at 580-680 ℃. The chemical reaction is the following [16]:
Al2O3+CsAlF4=Al2O3?CsAlF4 (17)
The composite molten salt which covers on the surface of Al-Li alloy uniformly protects the new surface of Al-Li alloy from being oxidized, which promotes the wetting and spreading of filler metal, thereby the brazing of Al-Li alloy is accomplished.
4 Conclusions
1) The oxide film on the surface of Al-Li alloy is mostly composed of Li2CO3 and amorphous Al2O3 at 500-540 ℃.
2) The CsF-AlF3 flux sufficiently reacts with the oxide film on the surface of Al-Li alloy at 500-540 ℃, which is the base of brazing of Al-Li alloy.
3) The flux CsF-AlF3 can effectively remove the oxide film by the way of reacting and/or dissolving the oxide film. The reacting residue are mostly Al2O3·CsAlF4, LiAlF4, CsLiAlF6 or Cs2LiAl3F12. CsF plays an important role in removing the oxide film. The activity of the flux is related to the compounds such as NH4F and NH4AlF4. The key step is the generation of HF, which induces and accelerates the reaction of removing oxide film. The generation of H2O also accelerates the reaction.
References
[1] RIOJA R J. Fabrication methods to manufacture isotropic Al-Li alloys and products for space and aerospace applications [J]. Materials Science and Engineering A, 1998, 257: 100-107.
[2] HUO Hong-qing, HAO Wei-xin, GENG Gui-hong. Development of the new aerocraft material—Aluminum-lithium alloy [J]. Vacuum and Cryogenics, 2005, 11: 63-69.
[3] YANG Shou-jie, LU Zheng, SU Bin. Development of aluminum- lithium alloys [J]. Journal of Materials Engineering, 2001, 5: 44-47. (in Chinese)
[4] LI Yan, DENG Ji-xiong, WEI Zuo-wei. Characteristics of oxide film on 1420 Al-Li alloy surface [J]. The Chinese Journal of Nonferrous Metals, 2002, 12(2): 369-373. (in Chinese)
[5] STROHMEIER B R, SCHRALL D M. Ion scattering analysis of speckles appearing on solution heat-treated Al-Li alloys [J]. Materials Letters, 1998, 37: 366-370.
[6] WANG Yong, HU Jie, HU Guo-ping. Welding research and development of weldable aluminum-lithium alloys [J]. Nonferrous Metals, 2002, 54: 16-18. (in Chinese)
[7] WANG Da-yong, FENG Ji-cai, WANG Pan-feng. Microstructures and mechanical properties of Al-Li alloy friction stir weld [J]. Acta Metallurgica Sinica, 2004, 40(5): 504-508. (in Chinese)
[8] ZHANG Qi-yun, ZHUANG Hong-shou. Manual of brazing and soldering [M]. Beijing: China Machine Press, 1998: 25-69. (in Chinese)
[9] GUO He-tong, WANG Wei. Retrospect and prospect of aluminium anodizing [J]. Materials Protection, 2000, 33: 43-45. (in Chinese)
[10] WEI Kun-xia, ZHAO Kun-yu, WEI Wei. Structural transformation of amorphous Al2O3 prepared by low-temperature combustion synthesis [J]. Ordnance Material Science and Engineering, 2005, 28: 41-43. (in Chinese)
[11] LIU Zhi-qiang. Preparation of high purity ultrafine aluminum oxide(Ⅱ)—Calcination influence on the property of aluminum oxide powder [J]. Journal of Guangdong Non-ferrous Metals, 2002, 12: 99-101. (in Chinese)
[12] PRASAD N H, BALASUBRAMANIAM R. Influence of laser surface treatment on the oxidation behaviour of an Al-Li-Cu alloy [J]. Journal of Materials Processing Technology, 1997, 68: 117-120.
[13] ZHANG Qi-yun, LIU Shu-qi, GAO Nian-zhong. The solution synthesis of potassium fluoaluminate flux and its brazing mechanism [J]. Transactions of the China Welding Institution, 1982, 3(4): 114-118. (in Chinese)
[14] ZHANG Qi-yun. Corrosion-free and non-dissolve flux for brazing aluminium and its alloy [J]. Welding & Joining, 1995, 10: 2-3. (in Chinese)
[15] XUE Song-bai, CHEN Wen-hua, L? Xiao-chun. Mechanism of brazing flux reacting with oxide film of LY12 aluminum alloy [J]. The Chinese Journal of Nonferrous Metals, 2004, 14: 543-547. (in Chinese)
[16] FIELD D J, STEWARD N I. Mechanistic aspects of the Nocolok flux brazing process [M]. Society of Automotive Engineers, 1988: 1656-1664.
[17] CHEN Rong, WU Gen-hua, ZHANG Qi-yun. Formation and thermodynamic stability of KAlF4 and related fluorides [J]. Chemistry, 2000, 9: 50-52. (in Chinese)
Foundation item: Project supported by the Open Fund of State Key Laboratory of Gansu Advanced Non-ferrous Metal Materials, Lanzhou University of Technology, China
Corresponding author: XUE Song-bai; Tel: +86-25-84896070; E-mail: xuesb@nuaa.edu.cn
(Edited by YUAN Sai-qian)