
Preparation for intermetallic powders of Cu-Sn and Cu-Ni-Sn systems via solid-liquid reaction milling technique
CHEN Ding(陈 鼎), WU Wei(吴 薇), CHEN Zhen-hua(陈振华),
FU Ding-fa(傅定发), CHEN Gang(陈 刚)
School of Materials Science and Engineering, Hunan University, Changsha 410082, China
Received 15 July 2007; accepted 10 September 2007
Abstract: The Cu-Sn binary intermetallic powders were obtained via a patented reaction ball milling technique. The Sn melt reacted with the solid-state Cu during the milling process at different temperatures for different intervals. Two kinds of binary intermetallics were obtained. For 12 h, Cu6Sn5 was prepared by milling Sn melt at 573 K while Cu3Sn by milling Sn melt at 773 K. And a mixture of Cu6Sn5 and Cu3Sn was fabricated at 673 K. All these intermetallic powders had mean grain sizes of less than 100 nm. A finer microstructure was obtained by milling Sn melt blended with 20%(mass fraction) Ni powders at 573 K for 12 h. The reaction mechanism and advantages were discussed in comparison with that of high-energy ball milling. The results show the solutionizing of Ni powders in the Cu6Sn5 intermetallic.
Key words: Cu-Sn binary alloy; intermetallics powder; solid-liquid reaction milling; mechanochemical effect
1 Introduction
The effects of external fields such as the magnetic field, the electric field and the force field on the solidification behavior of melts induced much finer and more uniform solidification microstructures[1-3]. As a result, the mechanochemical field, which combines the force field with the chemical field, has attracted much attention in recent years. Mechanochemical technique has been widely employed in preparing nano powder, nano composites and dispersion-strengthened structural alloy. As a dry, high-energy ball milling technique, mechanical alloying has been adapted to produce a variety of commercially useful and scientifically interesting materials such as supersaturates solid solutions, crystalline and quasicrystalline intermediate phases and amorphous alloys[4-6]. Solid-liquid reaction milling technique based on mechanochemistry and mechanical alloying is a novel technology for material preparing. Due to the effects of both aspects, good microstructures were obtained via this reaction ball milling technique. Up to now, pure binary intermetallic powders of Fe-Sn, Ti-Al, Ni-Al and Fe-Al systems such as FeSn, Fe3Sn2, Ti3Al, Ni2Al3 and Fe3Al, and ternary intermetallics of Al-Cu-Fe, Al-Fe-Si, Al-Cu-Co and Al-Cu-Ni alloy systems such as Al7Cu2Fe, Al13Cu4Fe3, Al65Cu20Fe15, Al8Fe2Si, Al3FeSi, Al65Co15Cu20, Al69Co25Cu6, Al7Cu4Ni and Al0.28Cu0.69Ni0-[7-11] have been successfully prepared. In this contribution, the Cu-Sn and Cu-Ni-Sn alloy systems were selected and binary intermetallics were prepared.
2 Experimental
The solid-liquid reaction milling process was conducted at the selected temperatures in a milling device (as shown in Fig.1), in which a sealed milling cylinder of d300 mm in diameter rotates in a resistance heated furnace equipped with the thermostatic system. Both the balls and the milling cylinder were made of the same starting material to prevent the melt from contamination.
Moreover, the whole device can be operated in vacuum or an inert gas atmosphere. Usually, the milling cylinder was evacuated first and then filled with pure inert gas to avoid oxidation.
The metal with the higher melting point such as Cu acted as the starting material, while the metal with the lower melting point such as Sn served as the reactant. The rotational speed was controlled at 80 r/min. The mass of the reactant was approximately 50 g. The mass ratio of the balls to the reactant was 20:1. The milling temperature was chosen higher than the melting point of the reactant based on Cu-Sn phase diagram[12] and thus it was in a molten state during the milling process. Powdery Ni with an average size of less than 74μm was added to study its effect on the reaction during the solid-liquid milling process. In addition, conventional mechanical alloying of Cu-Ni-Sn system was carried out in a high-energy planetary ball miller under the same conditions for comparison. The mass ratio of ball-to-powder was maintained 10:1 and the rotation speed was controlled at an angular velocity of 41.9 rad/s (i.e. 400 r/min). Blended powders of Cu, Sn and Ni served as the reactant.
The as-milled products were examined by X-ray diffraction (XRD), scanning electron microscopy (SEM) and transmission electron microscopy (TEM). For the X-ray diffraction we used Cu Kα radiation.
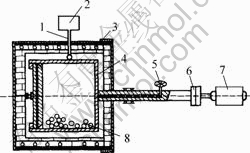
Fig.1 Schematic diagram of milling device: 1 Thermoelectric couple; 2 Thermostatic system; 3 Electric furnace; 4 Milling cylinder; 5 Vacuum value; 6 Shaft coupling; 7 Motor; 8 Milling ball
3 Results
3.1 Cu-Sn system
3.1.1 Milling Sn melt at 573 K
The as-milled products by milling Sn melt at different temperatures for different intervals are shown in Table 1. The XRD patterns are shown in Fig.2. After milling at 573 K for 6 h, Cu6Sn5 intermetallic was prepared. But there is still some Sn in the system. As seen from Fig.2(b), after milling for 12 h powders obtained are still Cu6Sn5 intermetallic and a little Sn.
3.1.2 Milling Sn melt at 673 K
As seen from Fig.3, the pure powders of Cu6Sn5 were obtained only after milling for 6 h. In comparison with the results above, the higher temperature accelerated the reaction. After milling for 12 h, the Cu3Sn intermetallic was prepared. But the powders obtained are a mixture of Cu6Sn5 and Cu3Sn intermetallics.
Table 1 As-milled products by milling Sn melt for different time at 573 K, 673 K and 773 K
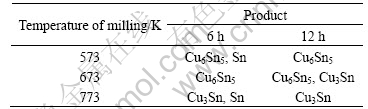
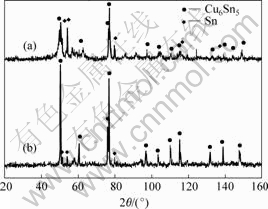
Fig.2 XRD patterns of as-milled products at 573 K for 6 h(a) and 12 h(b)
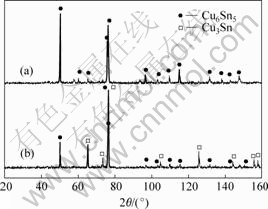
Fig.3 XRD patterns of as-milled products at 673 K for 6 h(a) and 12 h(b)
3.1.3 Milling Sn melt at 773 K
As seen from Table 1, Cu3Sn intermetallic already started to synthesize after milling for 6 h, and there is no Cu6Sn5 intermetallic. The pure Cu3Sn powders were obtained after milling for 12 h, as shown in Fig.4.
3.2 Cu-Ni-Sn system
3.2.1 Adding powders of Ni to Cu-Sn system at 573 K
Cu6Sn5 intermetallic powders were obtained by adding the powders of Ni to the Cu-Sn system at 573 K. No ternary alloy powders were fabricated. The XRD patterns of milling for 12 h is shown in Fig.5. The diffraction peak of Ni can not be found and the diffraction peak of Cu6Sn5 deviates to the high-angle orientation. The Ni powders must enter the Cu6Sn5 lattice.
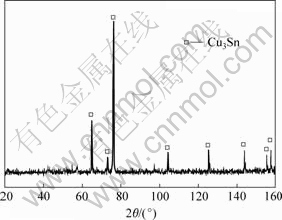
Fig.4 XRD pattern of as-milled product at 773 K for 12 h
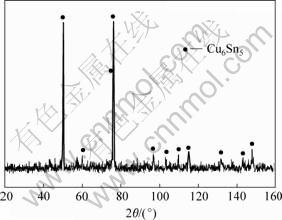
Fig.5 XRD pattern of as-milled product Cu6Sn5 by adding Ni powders to Cu-Sn system at 573 K for 12 h
3.2.2 Cu and Ni balls milling Sn melt at 573 K
The starting materials contained both the Cu and Ni balls. After milling the Sn melt at 573 K for 12 and 24 h, the as-milled products are Cu6Sn5 intermetallic powders. The XRD patterns for 12 h is shown in Fig.6. As seen from it, the diffraction peak of Ni still can not be found and the diffraction peak of Cu6Sn5 deviates to the high-angle orientation.
3.3 Microstructures of as-milled products
The SEM and TEM images of the as-milled products of the Cu-Sn and Cu-Ni-Sn systems are shown in Figs.7-9, which indicate that these powders are composed of agglomerated particles with a mean grain size of less than 100 nm. And as seen from Figs.7-9, a homogeneous and finer microstructure is obtained by adding the powders of Ni to Cu-Sn system.
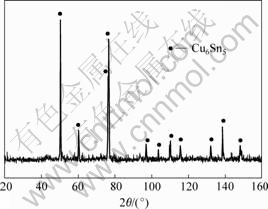
Fig.6 XRD pattern of as-milled product Cu6Sn5 at 573 K for 12 h with starting materials of both Cu and Ni balls
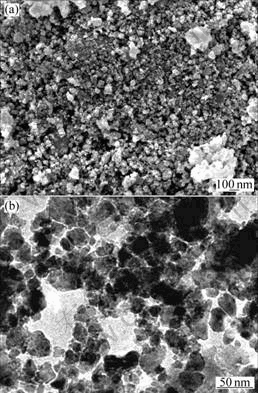
Fig.7 SEM(a) and TEM(b) images of Cu6Sn5 prepared by solid-liquid reaction milling
4 Discussions
4.1 Mechanism of solid-liquid reaction milling
The high-temperature phases of pure nanometer-sized intermetallic Cu6Sn5 and Cu3Sn were obtained via a solid-liquid reaction milling technique. The formation mechanism can be explained as follows. During milling processing, the newly formed reaction product on the surface of the balls peels off and breaks into fine particles during the continuous collisions among the balls and between the balls with the wall of the cylinder. The fresh surface when the intermetallic peels off helps to accelerate the solid-liquid reaction rate to form new intermetallic layer. These cycles will not stop until the melt is consumed. The added metal powders provide the larger reaction surface area to further accelerate the reaction rate. Finally, the formed particles deposit in the melt.
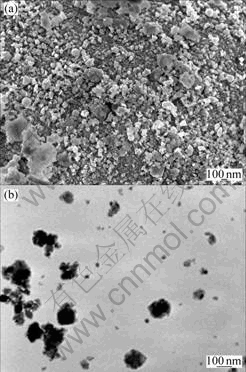
Fig.8 SEM(a) and TEM(b) images of Cu3Sn prepared by solid- liquid reaction milling
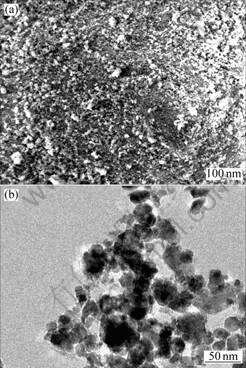
Fig.9 SEM(a) and TEM(b) images of Cu6Sn5 prepared by adding powders of Ni during solid-liquid reaction milling process
4.2 Effect of Ni powders
Addition of Ni powders to Cu-Sn system leads to a more uniform and finer microstructure. The solutionizing of Ni powders in Cu6Sn5 lattice (through a energy spectrum analysis) hinders the growth of the grain. On the other hand, the Ni powders may be smashed into smaller sizes in the process, which makes them possible to become the nucleating particles. And the increase of the number of the nucleating particles decreases the grain size.
4.3 Mechanically alloyed powders of Cu-Ni-Sn system
The XRD patterns powders prepared by conventional mechanical alloying are shown in Fig.10. After milling for 3 h, the diffraction peak of Sn disappears. And the diffraction intensity of Ni is weakened markedly after milling for 8 h. Then after milling for 20 h, all the Ni and Sn enter the Cu lattice. This is remarkably different from the experiment of Cu-Ni-Sn system above in which Ni powders enter the Cu6Sn5 intermetallic. The solid-liquid reaction milling involves a direct reaction while the mechanical alloying involves a kneading and diffusion of the powders.
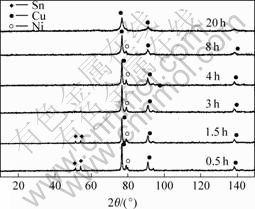
Fig.10 XRD patterns of as-milled products prepared by conventional mechanical alloying for different intervals
The experimental results show that the phase formation regularities during solid-liquid reaction mill processing is different from that of the conventional mechanical alloying methods, such as high-energy ball milling and tumbler milling. The former involves a direct reaction between the balls and the melt, while the latter is kneading and diffusion of powders in nature[4]. Consequently, solid-liquid reaction ball milling possesses a higher reaction speed than mechanical alloying, which is confirmed by the investigation result of other groups and the present study[13-14].
Furthermore, when alloy systems with intermetallic phases in the phase diagrams are treated by high-energy ball milling, amorphous alloys rather than intermetallics are obtained in most cases after milling for a long time and the intermetallics form directly in comparatively few alloy systems, In a sense, the solid-liquid reaction mill processing can result in an intensive solid-liquid reaction[6].
5 Conclusions
1) The high-temperature phases of pure Cu6Sn5 and Cu3Sn are obtained via a solid-liquid reaction milling route. The Cu6Sn5 and Cu3Sn also can be obtained during mechanical alloying, which needs much more time. Consequently, the reaction speed of solid-liquid reaction ball milling is higher than that of MA.
2) Addition of Ni powders to Cu-Sn system leads to a more uniform and finer microstructure, but does not change the phases obtained. It is found the solutionizing of Ni powders in Cu6Sn5 lattice.
References
[1] YOSHIAKIO O, SATO A. Grain refinement of solidification structure by ultrasonic vibration[J]. Casting Technology, 2000, 72(11): 733-738.
[2] LIU Jian-ming, LI Sheng-li, LI Jin, LIN Han-tong. Modification of solidification structure by pulse electric discharging[J]. Scripta, Metallurgical Materialia, 1994 , 31(12): 1691-1694.
[3] ASAI S. Birth and recent activities of electromagenetic processing of materials[J]. ISIJ Internationals, 1989, 29(12): 981-992.
[4] KOCH C C. Materials science and technology-A comprehensive treatment(Vol.15)[M]. Weinheim: VCH Verlagsgesellschaft GmvH, 1991: 193-345.
[5] SURYANARAYANA C. Bibliography on mechanical alloying and milling[M]. Cambridge: Cambridge International Science Publishing, 1995.
[6] CHEN Zhen-hua, CHEN Ding. Mechanical alloying and solid-liquid reaction ball milling[M]. Beijing: Chemistry Industry Press, 2006. (in Chinese)
[7] CHEN Ding, CHEN Ji-hua, YAN Hong-ge, CHEN Zhen-hua. Synthesis of binary and ternary intermetallic powders via a novel reaction ball milling technique[J]. Materials Science and Engineering A, 2007, 444: 1-5.
[8] CHEN Ding, CHEN Zhen-hua, CHEN Gang, HUANG Pei-yun. Preparation of the Al-Cu-Fe & Al-Fe-Si ternary intermetallic powders via a novel reaction ball milling technique[J]. Journal of Alloys and Compound, 2004, 376: 89-94.
[9] CHEN Zhen-hua, CHEN Ding. Preparation of binary and ternary intermetallic powdersvia a novel reaction ball milling technique[J]. Journal of Metastable and Nanocrystalline Materials, 2005, 24/25: 719-722.
[10] CHEN Zhen-hua, CHEN Ding, CHEN Gang, YAN Hong-ge, HUANG Pei-yun. Preparation of elevated-temperature intermetallic powders via a novel reaction ball milling technique[J]. Journal of Alloys and Compound, 2004, 370: 43-46.
[11] CHEN Ding, CAI Jian-guo, CHEN Gang-chen, YAN Hong-ge. Preparation of the Al-Cu-Co and Al-Cu-Ni ternary intermetallic powders via a solid-liquid reaction ball milling technique[J]. Material Science Forum, 2007, 561/565: 363-366.
[12] YU J Q. Binary phase diagrams[M]. Shanghai: Shanghai Science and Technology Press, 1987. (in Chinese)
[13] YANG Yuan-zheng, MA Xue-ming, DONG Yuan-da, ZHUANG Yu-zhi. Formation of Fe-Sn & Cu-Sn nano high-temperature phase under the mechanical drive[J]. Chinese Science Bulletin, 1994, 39 (17): 1626-1628. (in Chinese)
[14] LIU Xi, XIA Zhi-dong, LEI Yong-ping, SHI Yao-wu. The influence of milling conditions to the formation of Sn-0.7Cu alloy[J]. Transactions of the China Welding Institution, 2005, 26(5): 9-12. (in Chinese).
(Edited by YANG You-ping)
Foundation item: Project(50574039) supported by the National Natural Science Foundation of China
Corresponding author: WU Wei; Tel: +86-731-8821648; E-mail: wuvi2007@163.com