Influence of dioxin reduction on chemical composition of sintering exhaust gas with adding urea
来源期刊:中南大学学报(英文版)2012年第5期
论文作者:龙红明 李家新 王平
文章页码:1359 - 1363
Key words:sintering; dioxin; SO2; NOx; urea
Abstract:
With the addition of urea as an inhibitor, four groups of reducing dioxin emission experiments in sintering pot were conducted. The results show that, adding 0.05%, 0.1% and 0.5% (mass fraction) urea, the emission concentrations of dioxin are 0.287, 0.258 and 0.217 ng-TEQ/m3, respectively. The dioxin emission rates drop substantially compared to 0.777 ng-TEQ/m3 free of urea. With an increase of the urea content, the concentration of SO2 emission reduces sharply. (NH4)2SO4, formed by the reaction of SO2 and NH3, goes into the dust and part of NH3 is released before reaction with the emission of exhaust gas. The NOx emission presents an increasing trend because the reaction of NH3 and O2 at high temperature produces NOx. Based on the consideration of factors such as the effect of reducing dioxin emission, and the chemical composition of exhaust gas, 0.05% is the optimum adding content of urea.
J. Cent. South Univ. (2012) 19: 1359-1363
DOI: 10.1007/s11771-012-1150-y![]()
LONG Hong-ming(龙红明), LI Jia-xin(李家新), WANG Ping(王平)
School of Metallurgy and Resource, Anhui University of Technology, Ma’anshan 243002, China
? Central South University Press and Springer-Verlag Berlin Heidelberg 2012
Abstract: With the addition of urea as an inhibitor, four groups of reducing dioxin emission experiments in sintering pot were conducted. The results show that, adding 0.05%, 0.1% and 0.5% (mass fraction) urea, the emission concentrations of dioxin are 0.287, 0.258 and 0.217 ng-TEQ/m3, respectively. The dioxin emission rates drop substantially compared to 0.777 ng-TEQ/m3 free of urea. With an increase of the urea content, the concentration of SO2 emission reduces sharply. (NH4)2SO4, formed by the reaction of SO2 and NH3, goes into the dust and part of NH3 is released before reaction with the emission of exhaust gas. The NOx emission presents an increasing trend because the reaction of NH3 and O2 at high temperature produces NOx. Based on the consideration of factors such as the effect of reducing dioxin emission, and the chemical composition of exhaust gas, 0.05% is the optimum adding content of urea.
Key words: sintering; dioxin; SO2; NOx; urea
1 Introduction
Sintering plant is an important part of the entire production chain in iron and steel industry. It provides a large amount of low cost, stable quality sinter for the blast furnace. In recent years, the increasingly stringent regulatory requirements and market requirements of saving energy as well as reducing costs put double pressure on the sintering plant. The discharged pollutants of sintering plant mainly include particles, SO2, NOx, fluoride and dioxin. Among them, dioxin [1-3] is the most toxic persistent organic pollutant (POP) in unintentional synthesized byproducts. It is a tricyclic aromatic compound, composed by one or two oxygen atoms joining with two chloro-substituted benzene rings. It totally has 210 congeners, including polychlorinated dibenzo-p-dioxin (PCDD) and polychlorinated dibenzofuran (PCDF), which is collectively known as dioxin (PCDD/Fs). Dioxin detection is on ultra-trace level, lower by several magnitudes compared with other pollutants. More and more researches [4-8] show that the long-term hazards of dioxin are much more serious than we now realize. Therefore, the prevention of dioxin pollution should be paid great attention.
At present, high efficient flittering technology, physical absorbing technology, and catalytic decomposition are mainly adopted worldwide to reduce dioxin produced in the sintering process [9-15]. These methods are effective in some ways, but such problems as the complicated technology, the great renovation of process flow and the huge investment limit their application. Based on the analysis of the present research, the conclusion is drawn that it is more highly effective to develop the new technology inhabiting the release of dioxin in the sintering process than the traditional end treatment. However, study on the influence of new inhabiting technique on the chemical composition of sintering exhaust gas, and comprehensive evaluation of the feasibility of the technology based on emission reduction effect and its impact on the environment are seldom reported.
In this work, emission reduction effect and its impact on the environment are focused on. First, urea with different ratios is melted in water. Then, the water is added to the raw materials when the mixture is blended in the mixer. With the sintering process going on, urea is discomposed by heating, and ammonia is produced in the drying and pre-heating zone. With a series of chemical reactions, the production of dioxin is inhabited. The optimum proportion of adding urea to inhibit the dioxin emission is obtained with the sintering pot experiments. Further, the influence on the chemical composition of exhaust gas in sintering process is studied to ascertain whether it increases the release of other pollutants (SO2 and NOx) by addition of urea. This research will provide basic experiment data for developing high efficiency and low cost dioxin emissions reduction technology in sintering process.
2 Experimental
2.1 Material properties
The sintering material came from the raw material of the sintering plant of an iron and steel company. The ferrous raw materials mainly included the ore powder (Newman, Kara, Yankee and FMG) and metallurgy accessories (blast furnace dust, and rolling skin). Flux mainly included lime, limestone, dolomite, and fuel includes coal and coke. The chemical compositions of raw materials are listed in Table 1. In the experiments, a fixed ratio of raw materials was adopted, as listed in Table 2. The urea used in the experiments is the common industrial urea, in which nitrogen content is larger than 46.3%, and particle size ranges 0.85-2.80 mm. The urea was dissolved and added with water in the raw material and granulating process. The urea mixing ratio in four experiments is given in Table 3.
2.2 Methods
2.2.1 Parameter of sintering
Sintering process occurred in the sintering pot, of which the diameter is 150 mm, the height of the material bed is 600 mm, the ignition time is 2 min, the ignition temperature is 1 100 °C, the negative pressure is 12 kPa, and the hearth layer mass is 2 kg. A pumping pore of about 30 mm in diameter is opened on the exhaust gas pipeline, and is kept sealed. The schematic diagram of sintering pot is shown in Fig. 1. Each instance of tests in one group was repeated three times, and all data are the average values of them.
2.2.2 Analysis methods of dioxin, ammonia and exhaust gas
Dioxin sample collection started from sintering ignition and ended at the BTP (Burn Through Point), which is the top point of the exhaust gas temperature curve. Samples were collected in the filter membrane and adsorption materials. The sample was removed and saved. The isotope dilution high resolution gas chromatography-high resolution mass spectrometry (HRGC-HRMS) was employed to detect the dioxin. The collected sample was added with isotope-labeled internal sign. Sample extraction liquid was acquired for dealing with filter membrane and adsorption materials, and then it was purified and concentrated into final analytical sample.
The method of Nessler spectrophotometry was used to detect ammonia, with precision of 0.03 mg/m3. The portable flue gas analyzer KM9406E produced by British Kane Company was used to analyze the contents of O2, CO, SO2, NOx in exhaust gas. A set of data was recorded automatically every 20 s.
Table 1 Chemical compositions of raw materials (mass fraction, %)
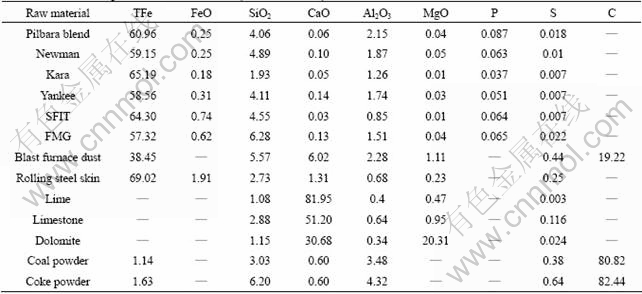
Table 2 Ratio of raw materials (mass fraction, %)

Table 3 Urea mixing ratio (mass fraction, %)
![]()
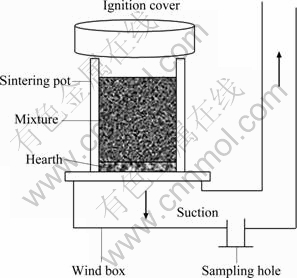
Fig. 1 Schematic diagram of sintering pot
3 Results and analysis
3.1 Effect on dioxin reduction
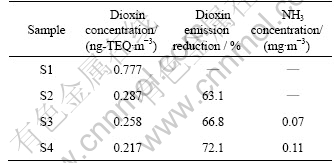
The results show that in 17 kinds of homologues of dioxin, the discharge concentration of dioxin in the exhaust gas of sintering pot without adding urea is 0.777 ng-TEQ/m3; with adding 0.05%, 0.1%, 0.5% urea, it is 0.287, 0.258 and 0.217 ng-TEQ/m3, respectively, as listed in Table 4. The reducing effect is remarkable. The dioxin emissions are decreased by 63.1%, 66.8% and 72.1% compared with free urea, which illustrates that urea has a significant inhibitory effect on the formation of dioxin in sintering process. With increasing the ratio of urea, the reduction also increases, but the increasing trend gradually decreases. For instance, the urea content of the fourth group is 10 times of the second one, but the reduction is increased by only 9.0%. The reason is that the function of inhibiting the formation of dioxin by urea mainly depends on the low-temperature synthesis reactions. Stable nitride forms through the reaction of ammonia and Cu2+, reducing the catalytic activity of Cu2+, and through the reaction of ammonia and HCl, reducing the chlorine source of dioxin formation. For the high temperature gas synthesis reaction, urea has no obvious inhibition effect; in contrast, the HCl forming in the high-temperature reaction may lead to the increase of dioxin concentration from the low-temperature synthesis reactions [16].
Table 4 Analyzing results of dioxin emission
3.2 Analysis of O2 and CO contents
The contents of O2 and CO in the sintering exhaust gas in the experiments are shown in Fig. 2 and Fig. 3, respectively. With an increase of the urea ratio, O2 presents an increase trend while the concentration of CO slightly decreases. The phenomenon displays the improvement of the fuel utilization efficiency. Combined with sintering technical index, it also proves the efficiency improvement. Vertical sintering speed gradually increases from 23.08 to 26.09 mm/min, sinter utilization factor rises from 1.49 to 1.70 t/(h·m2), but drum strength decreases from 62.19% to 58.54%. Therefore, in the view of the negative influence of sinter drum strength, the suitable ratio of urea should not be higher than 0.05%.
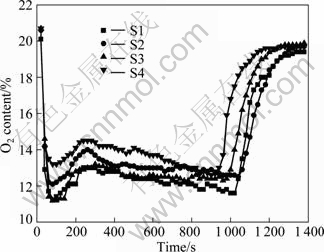
Fig. 2 O2 content in sintering exhaust gas
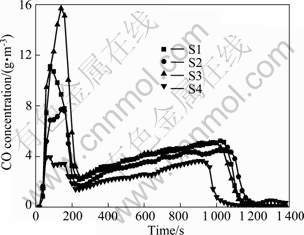
Fig. 3 CO concentration in sintering exhaust gas
3.3 Analysis of SO2 concentration
The concentration of SO2 in sintering exhaust gas is shown in Fig. 4. The concentration of SO2 varies obviously. In the sintering process, there are two clear peaks. The first peak appears when igniting, where the SO2 comes from ignition gas. The second peak emerges when wet material zone disappears in sintering material layer and the temperature in lower part of material bed rises quickly. It is the critical point to release SO2 in sintering exhaust gas. With an increase of urea adding content, the two peaks decrease gradually especially for the second one, which even disappear in S3 and S4 samples. The results indicate that urea does have remarkable effect on SO2 emission reduction.
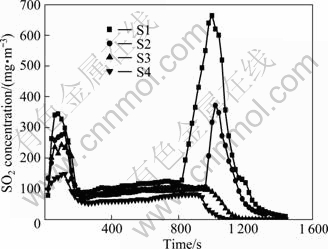
Fig. 4 SO2 concentration in sintering exhaust gas
The above analysis indicates that with the temperature rising in material layer, urea and SO2 may react in the way [17]:
(NH2)2CO+H2O=NH4COONH2 (1)
NH4COONH2=2NH3+CO2 (2)
SO2+2NH3+H2O+0.5O2=(NH4)2SO4 (3)
The reaction of SO2 and NH3 produces (NH4)2SO4. Gibbs free energy increases with the temperature rising. Under standard conditions, when the temperature is above 800 K (527 °C), the reaction cannot proceed to the right. This illustrates that in sintering process, reaction (3) mainly occurs in the middle-lower part of material layer bed during the period before the sintering BTP arrives. During this period, the relatively higher negative pressure and the slow flow speed of exhaust gas make the reaction fully-reacted. It is shown that SO2 concentration varies with time, which coincides with the experiment results.
There is absolutely no (NH4)2SO4 in sinter because when the temperature is higher than 1 200 °C, liquid phase forms consolidated sinter, and (NH4)2SO4 should be completely decomposed. Besides, (NH4)2SO4 may go to dust. Sintering dust in dust catcher and dust left in exhaust fan rotor are collected, and soaked in water. The analysis result of the chemical components in water shows that the sulfate radical contents of dust are 0.59%, 3.98%, 4.52%, and 5.66%, respectively, in S1, S2, S3 and S4 samples. The amount is far lower without adding urea compared with adding urea. With adding more urea, sulfate radical content still presents an increasing trend but with lower speed. Therefore, it can be inferred that reaction (3) occurs. Further analysis indicates that after the reaction of SO2 and NH3 in material layer, (NH4)2SO4 is formed and goes into wet material zone in the lower part of sintering bed with exhaust gas. Water is so sufficient that (NH4)2SO4 can be intercepted and dissolved in water. With the temperature rising in material layer, permeability becomes better, and (NH4)2SO4 is precipitated and attached to dust, and then is blown out of material layer with high-speed exhaust gas. Before the high temperature arrives the bottom of sintering bed, it exists in exhaust gas pipeline, avoiding decomposition [18].
Furthermore, NH3 of exhaust gas in each group is analyzed and the result is listed in Table 4. It can be seen from the result that when the adding ratios of urea are 0 and 0.05%, there is no emission of ammonia in exhaust gas; when the adding ratios of urea are 0.1% and 0.5%, the emission concentrations are 0.07 and 0.11 mg/m3, respectively. So, if the addition of urea is excessive, part of NH3 before reaction will go right into the exhaust gas pipeline and escape with the release of exhaust gas, which is undesired obviously. As ammonia is a hazardous and harmful gas to human beings, the emission will undoubtedly increase the environment burthen, leading to the secondary pollution.
3.4 Analysis of NOx content
The concentration of NOx in sintering exhaust gas is shown in Fig. 5. During middle time of sintering process, the curve presents a regular trend with a platform. The difference is that the platforms of S1 and S2 samples are essentially coincident, while the platforms of S3 and S4 samples present a significant rising trend, from an average of 200 to 230 and 275 mg/m3, respectively. NOx synthesis is closely related to the temperature. When the temperature rises in the range of 760-840 °C in material layer, reaction (4) instantly occurs. From the perspective of the sintering process, this temperature range just appears in sintering middle time and maintains for a period of time. If O2 is sufficient, reaction (5) further occurs, from which NO2 is formed. Thus, the NOx concentration platform rises obviously, but the emission regularity keeps nearly the same.
4NH3+5O2=4NO+6H2O (4)
2NO+O2=2NO2 (5)
As for sintering techniques and environmental protection, the higher concentration of NOx emission is undesirable. Thus, the amount of urea addition should not be higher than 0.05%.
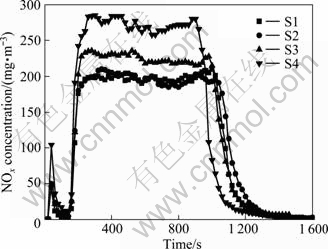
Fig. 5 NOx concentration in sintering exhaust gas
4 Conclusions
1) Adding urea is effective to reduce the dioxin emission in sintering process. While adding 0.05%, 0.1% and 0.5% urea, the dioxin emission is 0.287, 0.258 and 0.218 ng-TEQ/m3, respectively. That is, the dioxin emission decreases by 63.1%, 66.8% and 72.1% compared to that free of urea. This illustrates that urea has an extraordinary inhibition effect on the dioxin formation in sintering process.
2) Though addition of urea has little influence on the concentrations of O2 and CO, it influences SO2 and NOx significantly. With the increase of urea ratio, the two peaks of SO2 concentration curve gradually disappear. (NH4)2SO4 formed by the reaction of SO2 and NH3 goes into dust. Part of NH3 is released with exhaust gas emission before reaction. The curve platform of NOx emission concentration presents a rising trend because of the reaction of NH3 and O2 at high temperature.
3) Based on the consideration of different factors such as reducing dioxin emission effect, the influence of sinter performance parameters and chemical composition of exhaust gas, 0.05% (mass fraction) is the optimum adding ratio of urea.
References
[1] CHEN Yu-cheng, TSAI Perng-jy, MOU Jin-luh. Determining optimal operation parameters for reducing PCDD/F emissions (I-TEQ values) from the iron ore sintering process by using the Taguchi experimental design [J]. Environ Sci Technol, 2008, 42: 5298-5303.
[2] BOSCOLO M, PADOANO E, TOMMASI S. Identification of possible dioxin emission reduction strategies in pre-existing iron ore sinter plants [J]. Ironmaking and Steelmaking, 2008, 35(2): 146-152.
[3] MENDA N, TAYIBI H, FEMANDO G C, HERMANDEZ A. Minimization methods for emissions generated from sinter strands: A review [J]. Journal of Cleaner Production, 2006, 14: 740-747.
[4] STANMORE B R. The formation of dioxins in combustion systems [J]. Combustion and Frame, 2004, 136(3): 398-427.
[5] PRASHANT S.K, JOAO G. C, CARLOS A M A. Dioxins sources and current remediation technologies—A review [J]. Environment International, 2008, 34: 139-153.
[6] ISHII K, FURUICHI T. Development of bioreactor system for treatment of dioxin-contaminated soil using pseudallescheria boydii [J]. Journal of Hazardous Materials, 2007, 148(3): 693-700.
[7] MASANORI N, KAZUYUKI M, TAKEHIKO S. Factors accelerating dioxin emission from iron ore sintering machines [J]. ISIJ Int, 2009, 49(5): 729-734.
[8] SHUNJI K, YUICHI Y, KAZUOMI W. Investigation on the dioxin emission from a commercial sintering plant [J]. ISIJ Int, 2006, 46(7): 1014-1019.
[9] MASANORI N, YOHZOH H, EIKI K. Observation of behavior of dioxins and some relating elements in iron ore sintering bed by quenching pot test [J]. ISIJ Int, 2005, 45(4): 609-617.
[10] EIKI K, SHUNSUKE K, HIROSHI G, TAICHI M. Reduction in dioxin emissions by the addition of urea as aqueous solution to high-temperature combustion gas [J]. ISIJ Int, 2008, 48(9): 1305-1310.
[11] CHENG He-fa, HU Yua-nan. Curbing dioxin emissions from municipal solid waste incineration in China: Re-thinking about management policies and practices [J]. Environmental Pollution, 2010, 158(9): 2809-2814.
[12] CELINE X, EDWIN D P. Prevention of dioxins de novo formation by ethanolamines [J]. Environ Chem Lett, 2003, 1: 51-56.
[13] FICARELLA A, LAFORGIA D. Numerical simulation of flow-field and dioxins chemistry for incineration plants and experimental investigation [J]. Waste Management, 2000, 20: 27-49.
[14] VALERIE M T, COLIN M M. Relation of chlorine, copper and sulphur to dioxin emission factors [J]. Journal of Hazardous Materials, 2008, 151(1): 164-170.
[15] ANDERSON D R, FISHER R. Sources of dioxins in the United Kingdom: The steel industry and other sources [J]. Chemosphere, 2002, 46: 371-381.
[16] LONG Hong-ming, LI Jia-xin, WANG Ping, GAO Gang, ZHANG Jian. Reaction mechanism of emission of dioxin by addition of urea in iron ore sintering process [J]. The Chinese Journal of Process Engineering, 2010, 10(5): 944-949.
[17] LIAO Ji-yong, BI Xue-gong, XIONG Wei, JIN Yan. Simulation research for sintering waste gas desulfurization [J]. Sintering and Pelletizing, 2006, 31(6): 4-8.
[18] BI Xue-gong, LIAO Ji-yong, XIONG Wei, ZHOU Gou-fan, FENG Zhi-hui. Experimental study of SO2 and NOx removal during sintering process [J]. Journal of Wuhan University of Science and Technology, 2008, 31(5): 449-452.
(Edited by YANG Bing)
Foundation item: Project(50904001) supported by the National Natural Science Foundation of China; Project(2010SQRL032D) supported by Anhui Provincial Key Science Foundation for Outstanding Young Talent, China; Project(TD200909) supported by Program for Innovative Research Team in Anhui University of Technology, China
Received date: 2011-03-15; Accepted date: 2011-05-11
Corresponding author: LONG Hong-ming, Associate Professor, PhD; Tel: +86-555-2311571; E-mail: yaflhm@126.com

