锶对挤压态二元锌锶合金显微组织、力学性能及体外降解行为的影响
来源期刊:中国有色金属学报(英文版)2020年第7期
论文作者:柯贵州 岳锐 黄华 康斌 曾晖 袁广银
文章页码:1873 - 1883
关键词:锌锶合金;挤压;组织;力学性能;体外降解行为
Key words:Zn-Sr alloy; extrusion; microstructure; mechanical properties; in vitro degradation behavior
摘 要:系统研究纯锌和 Zn-xSr (x=0.1%, 0.4%, 0.8%, 质量分数)合金热挤压后的显微组织、力学性能及体外降解行为。研究发现:添加 0.1% Sr 后合金中析出SrZn13相,使挤压后合金的屈服强度、抗拉强度及伸长率由纯锌的(85.33±2.86) MPa、(106.00±1.41) MPa和(15.37±0.57)%分别提高到(107.67±2.05) MPa、(115.67±2.52) MPa和(20.80±2.19)%;继续增加Sr含量,由于析出粗大的SrZn13相,容易引用应力集中和裂纹产生,使合金的性能下降。此外,分布不均匀的SrZn13相与基体之间存在的微电偶腐蚀作用使得添加Sr后合金的体外降解速度加快,同时,降解变得不均匀。随着Sr含量的增加,降解速度逐渐加快,由纯锌的(11.45±2.02) mm/a逐渐增加至 Zn-0.8Sr的(32.59±3.40) mm/a。其中,挤压态Zn-0.1Sr 合金具有最佳的综合力学性能与降解性能。
Abstract: The microstructures, mechanical properties and in vitro degradation behavior of as-extruded pure Zn and Zn-xSr (x=0.1, 0.4, 0.8 wt.%) alloys were investigated systematically. For the microstructure and mechanical properties, SrZn13 phase was newly formed due to the addition of 0.1 wt.% Sr, improving the yield strength, ultimate tensile strength and elongation from (85.33±2.86) MPa, (106.00±1.41) MPa and (15.37±0.57)% for pure Zn to (107.67±2.05) MPa, (115.67±2.52) MPa and (20.80±2.19)% for Zn-0.1Sr, respectively. However, further increase of Sr content led to the deterioration of the mechanical properties due to the stress concentration and cracks initiation caused by the coarsening SrZn13 particles during tensile tests. For in vitro degradation, since micro galvanic corrosion was enhanced owing to the formation of the inhomogeneously distributed SrZn13 phase, the corrosion mode became non-uniform. Corrosion rate is gradually increased with the addition of Sr, which is increased from (11.45±2.02) μm/a (a=year) for pure Zn to (32.59±3.40) μm/a for Zn-0.8Sr. To sum up, the as-extruded Zn-0.1Sr alloy exhibited the best combination of mechanical properties and degradation behavior.
Trans. Nonferrous Met. Soc. China 30(2020) 1873-1883
Gui-zhou KE1, Rui YUE1, Hua HUANG1,2, Bin KANG3, Hui ZENG3, Guang-yin YUAN1,2
1. National Engineering Research Center of Light Alloy Net Forming and Key State Laboratory of Metal Matrix Composites, School of Materials Science and Engineering, Shanghai Jiao Tong University, Shanghai 200240, China;
2. Shanghai Innovation Institute for Materials, Shanghai 200444, China;
3. Department of Orthopedics, Peking University Shenzhen Hospital, Shenzhen 518036, China
Received 11 September 2019; accepted 18 May 2020
Abstract: The microstructures, mechanical properties and in vitro degradation behavior of as-extruded pure Zn and Zn-xSr (x=0.1, 0.4, 0.8 wt.%) alloys were investigated systematically. For the microstructure and mechanical properties, SrZn13 phase was newly formed due to the addition of 0.1 wt.% Sr, improving the yield strength, ultimate tensile strength and elongation from (85.33±2.86) MPa, (106.00±1.41) MPa and (15.37±0.57)% for pure Zn to (107.67±2.05) MPa, (115.67±2.52) MPa and (20.80±2.19)% for Zn-0.1Sr, respectively. However, further increase of Sr content led to the deterioration of the mechanical properties due to the stress concentration and cracks initiation caused by the coarsening SrZn13 particles during tensile tests. For in vitro degradation, since micro galvanic corrosion was enhanced owing to the formation of the inhomogeneously distributed SrZn13 phase, the corrosion mode became non-uniform. Corrosion rate is gradually increased with the addition of Sr, which is increased from (11.45±2.02) μm/a (a=year) for pure Zn to (32.59±3.40) μm/a for Zn-0.8Sr. To sum up, the as-extruded Zn-0.1Sr alloy exhibited the best combination of mechanical properties and degradation behavior.
Key words: Zn-Sr alloy; extrusion; microstructure; mechanical properties; in vitro degradation behavior
1 Introduction
Zn-based alloys have attracted increasing attention as potential biodegradable materials for orthopedic implants, cardiovascular inter- ventional devices and tissue engineering scaffold applications [1-3], thanks to their acceptable bio- compatibility, biodegradability [4], and appropriate degradation behavior [5,6]. Zn element plays an indispensable role in the nucleic acid metabolism and cell differentiation in the human body as an essential trace element, which includes DNA polymerase, RNA polymerase and many transcription factors [7]. For adults, the recommended intake of Zn element is 15 mg/d, and the human body is able to absorb Zn element from daily food, transport it along the vessels and excrete excess amounts through the kidneys [8]. BOWEN et al [9] observed the firm clinging tissue to the zinc wire and new bone formation nearby the zinc pins after they planted those implants into abdominal aorta and femora of mice for 180 d, indicating the participation of Zn implants in tissue recovery with no significant toxicity. The advantages of Zn as potential biodegradable devices rely upon its appropriate electrode potential (-0.762 V), which lies between that of Mg (-2.372 V) and that of Fe (-0.444 V) [10]. The degradation rate of pure Zn will be slower than that of Mg and faster than that of Fe. However, pure Zn is not suitable for application as biodegradable material due to its poor mechanical properties.
Alloying would be a feasible method to optimize the performance of Zn in mechanical properties and degradation behavior. In the past few years, Mg, Ca, Sr, Mn, Cu and Fe have been introduced into the Zn to develop biodegradable Zn-based alloys [11-19]. Alloying with 1 wt.% Mg, Ca or Sr for as-cast pure Zn could enhance its degradation rate, tensile strength and hardness by nearly 100% [16]. Alloying with Cu could also significantly enhance its degradation rate and mechanical properties [15]. Furthermore, alloying of Fe into the Zn-Cu system exhibited significant improvement in degradation rate, which exceeded 60 μm/a (a=year) in c-SBF solution [17]. The Mn addition also contributed to the properties improvement of Zn alloys [18,19]. Among those alloying elements, Sr is one of the essential trace elements for human body, which plays an important role in the growth of osteoblasts and restraint of the activity of osteoclast, as well as the adjustment of calcium metabolism [20]. All those physiological activities mainly take place in the bone and teeth, and excessive metabolites of Sr could transport out of the human body by circulation system [21,22]. Thus, the addition of Sr into Zn alloys may be enhanced the biological functions. LI et al [16] studied the Zn-1Sr binary alloy in the aspects of mechanical properties, corrosion behavior and biocompatibility. It was reported that the ultimate tensile strength of as-cast Zn-1Sr reached (171.40±14.13) MPa, while the elongation was (2.03±0.22)% and corrosion rate of as-rolled Zn-1Sr alloy exceeded 90 μm/a in Hanks’ solution. The Zn-1Sr alloy also exhibited good biological compatibility in vitro and in vivo. The microstructure and properties of as-cast Zn-xSr (x=0, 0.5, 1, 2, 3 wt.%) alloys were further studied by LIU et al [23]. The results show that the hardness and corrosion rate increased with the addition of Sr. Though most of the implants were fabricated by wrought materials there has not been research about the effect of the Sr content on the properties of as-extruded Zn-Sr binary alloys.
In this study, the microstructure, mechanical properties and in vitro degradation behavior of the as-extruded Zn-Sr binary alloys were investigated systematically. Since the elongation was only (2.03±0.22)% for as-cast Zn-1Sr alloy [16], three Zn-xSr (x=0.1, 0.4, 0.8 wt.%) binary alloys with the Sr content lower than 1 wt.% were designed so as to further improve the deformability. This research could be a reference for future research on biodegradable Zn alloys containing Sr.
2 Experimental
2.1 Alloys preparation
The designed Zn-xSr (x=0.1, 0.4, 0.8 wt.%) binary alloys were prepared with pure Zn (99.995 wt.%) and pure Sr (99.99 wt.%). Pure Zn was firstly melted at 620 °C in the steel crucible with pretreatment of coating inside of it, and then immersed the pure Sr (wrapped in the Zn foil) into the melt, under the protection of high purity argon. The melt was kept at 650 °C for 1 h, followed by refining with hexachloroethane. After the temperature cooling down to 600 °C, the melt was cast into a cylinder steel mold. The as-cast ingots and pure Zn (99.995 wt.%) were pre-processed into glossy cylinder billet before hot extrusion at 180 °C with an extrusion ratio of 9:1.
2.2 Microstructure analysis
Inductively coupled plasma atomic emission spectroscopy (ICP-AES, iCAP7600) was applied for the characterization of the actual chemical compositions of the as-cast alloys, and the results were listed in Table 1. There was only a slightly difference (no more than 0.03 wt.%) between the nominal composition and chemical composition. Optical microscope (OM, Zeiss Axio Observer A1, Germany) was used to observe the microstructure of the alloy specimens cut from as-cast ingots and as-extruded rods. All those specimens were ground and polished before etching by chromic acid (1 L distilled water, 50 g chromium trioxide, and 15 g sodium sulfate). Physical phase constitution analysis was investigated by X-ray diffraction (XRD, D/MAX2550, Rigaku Corporation, Japan) with Cu Kα radiation at 100 mA, 40 kV, and scanning rate of 5 (°)/min within the 2θ range from 20° to 90°. The scanning electron microscope (SEM, Sirion-200, FEI, USA) equipped with energy- dispersive spectrometry (EDS) was adopted for the observation of the second phase, the fracture surface after the tensile test and the samples with and without degradation products after immersion test.
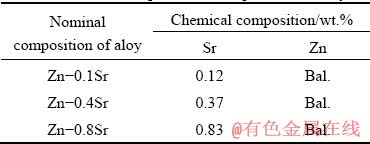
Table 1 Chemical compositions of experimental alloys
2.3 Mechanical test
The samples for tensile test were cut from as-extruded rods along the extrusion direction with a gauge length of 10 mm. All the samples were ground with 3000 grit SiC paper before conducting the test on the universal testing machine (Zwick/Roell Z100, Germany). The tensile speed was 1.2 mm/min.
2.4 Immersion test
All the samples were prepared by grinding with 3000-grit SiC paper and ultrasonic cleaning for 10 min. According to ASTM G31—72 [24], simulated body fluid (SBF) solution was prepared for the immersion test. Each sample was immersed into the SBF solution at (37±0.5) °C for 20 d, and the solution was renewed every 2 d. SBF solution volume to sample surface area ratio was 30 mL/cm2. After the immersion test, all the samples were ultrasonic cleaned for 10 min again and washed with chromic acid (1 L distilled water containing 200 g chromium trioxide) to remove the degradation products. The in vitro corrosion rate was measured by the following equation [24]:
ηCR=KW/(ATD) (1)
where ηCR (μm/a) represented the corrosion rate, K was a constant of 8.76×107, W (g) was the mass loss, A (cm2) was the sample’s surface area, T (h) was the immersion time and D (g/cm3) was the density of each sample.
3 Results
3.1 Microstructure and phase composition
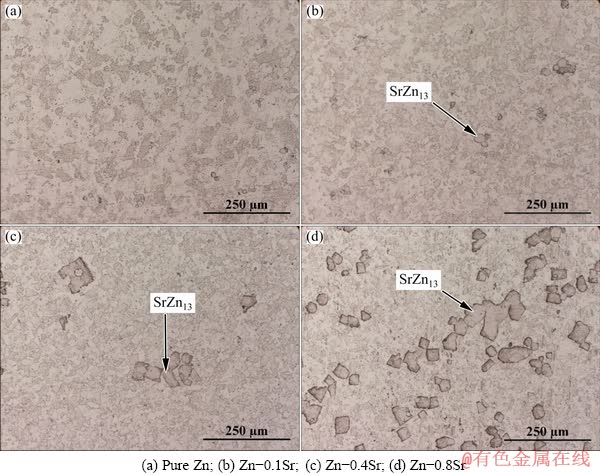
Fig. 1 Optical microstructures of as-cast pure Zn and Zn-xSr (x=0.1, 0.4, 0.8 wt. %) alloys
Optical microstructures (OM) of as-cast pure Zn and Zn-xSr (x=0.1, 0.4, 0.8 wt.%) alloys were shown in Fig. 1. With the increase of Sr content, more precipitates could be observed. The average size of the precipitated particles was (31.73± 4.35) μm for Zn-0.1Sr alloy, (40.26±9.77) μm for Zn-0.4Sr alloy and (46.09±11.67) μm for Zn-0.8Sr alloy. These precipitates appeared to distribute randomly in the Zn matrix in a sharp rectangular shape. The XRD patterns of as-cast Zn-xSr alloys (x=0.1, 0.4, 0.8 wt.%) were presented in Fig. 2, revealing that SrZn13 phase was a newly formed second phase due to the addition of Sr. However, the peak of SrZn13 phase for Zn-0.1Sr alloy was not as obvious as that of Zn-0.8Sr, which may be attributed to the much smaller amount of Sr in Zn-0.1Sr alloy. To further identify the precipitates observed in Fig. 1, SEM and EDS studies were performed for the Zn-0.4Sr alloy sample and the results were shown in Fig. 3. The rectangular precipitated particle only consisted of Zn and Sr with a molar ratio of nearly 13:1. Taking the XRD results into consideration, the rectangular precipitated particles could be identified as SrZn13 phase.
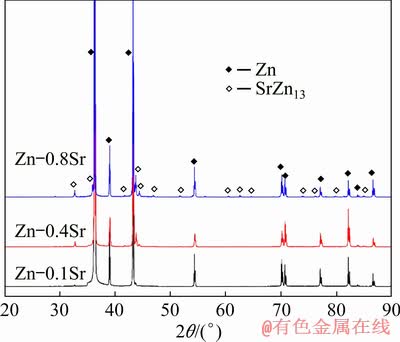
Fig. 2 XRD patterns of as-cast Zn-xSr (x=0.1, 0.4, 0.8 wt.%) alloys

Fig. 3 SEM image and EDS results of as-cast Zn-0.4Sr alloy
Figure 4 showed the optical microstructures of as-extruded pure Zn and Zn-xSr (x=0.1, 0.4, 0.8 wt.%) alloys. After hot extrusion, only a small amount of SrZn13 phase was broken into pieces with sharp edges, while most of SrZn13 particles remained sharp rectangle shape with nearly the original size. The average size of the SrZn13 phase after extrusion was (27.25±6.21) μm for Zn-0.1Sr alloy, (34.05±5.73) μm for Zn-0.4Sr alloy and (41.35±12.34) μm for Zn-0.8Sr alloy, respectively. In addition, the grain size of Zn matrix slightly decreased as the Sr content increased. The average grain size of Zn matrix after extrusion was (29.50±5.83) μm for pure Zn, (20.10±6.18) μm for Zn-0.1Sr alloy, (18.30±4.95) μm for Zn-0.4Sr alloy and (18.02±4.90) μm for Zn-0.8Sr alloy. Collectively, the studied alloys were recrystallized completely and SrZn13 phase remained almost unchanged after hot extrusion. At the same time, grains close to the SrZn13 phase exhibited smaller size because of the particle-stimulated nucleation (PSN) during the recrystallization [25].
3.2 Mechanical properties
The mechanical properties of as-extruded pure Zn and Zn-xSr (x=0.1, 0.4, 0.8 wt.%) alloys were shown in Fig. 5. Figure 5(a) showed the representative stress-strain curves, and Fig. 5(b) exhibited the statistical results of yield strength (YS), ultimate tensile strength (UTS) and elongation. The YS was (85.33±2.86) MPa for pure Zn, (107.67±2.05) MPa for Zn-0.1Sr alloy, (95.00±2.45) MPa for Zn-0.4Sr alloy, and (90.67±1.70) MPa for Zn-0.8Sr alloy. The UTS was (106.00±1.41) MPa for pure Zn, (115.67±2.52) MPa for Zn-0.1Sr alloy, (102.33±1.53) MPa for Zn-0.4Sr alloy, and (95.10±2.89) MPa for Zn-0.8Sr alloy, correspondingly. The elongation was (15.37±0.57)% for pure Zn, (20.80±2.19)% for Zn-0.1Sr alloy, (14.67±1.63)% for Zn-0.4Sr alloy, and (12.67±1.42)% for Zn-0.8Sr alloy. The as-extruded Zn-0.1Sr alloy showed the most desirable combination of YS, UTS, and elongation.
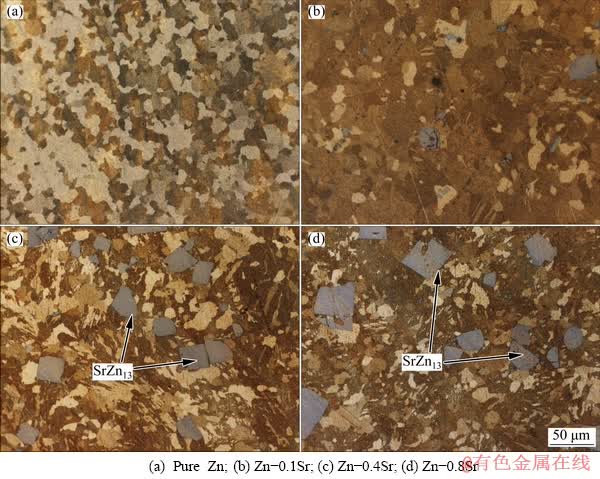
Fig. 4 Optical microstructures of as-extruded pure Zn and Zn-xSr (x=0.1, 0.4, 0.8 wt.%) alloys
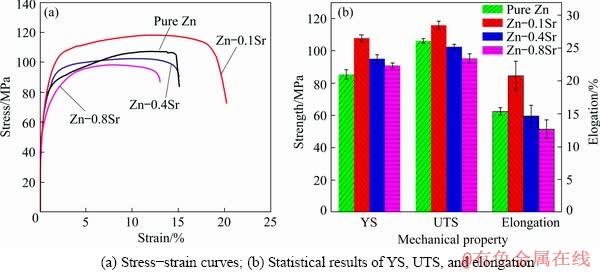
Fig. 5 Mechanical properties of as-extruded pure Zn and Zn-xSr (x=0.1, 0.4, 0.8 wt.%) alloys
Figure 6 shows the fracture surface morphologies of as-extruded pure Zn and Zn-Sr alloys after tensile test. River patterns in different cleavage planes were obviously observed in pure Zn (Fig. 6(a)), indicating the brittle fracture during the tensile test. Similarly, cleavage fracture characteristics were also observed in Figs. 6(b-d) for Zn-Sr binary alloys, indicating the typical cleavage fracture mode for Zn-Sr alloys. In comparison, Zn-0.1Sr alloy was a little different from the others with a few dimples which could be observed in Fig. 6(b). Therefore, with the increasing addition of Sr to more than 0.1 wt.%, the deformability of Zn-Sr binary alloys decreased correspondingly.
3.3 Degradation behavior
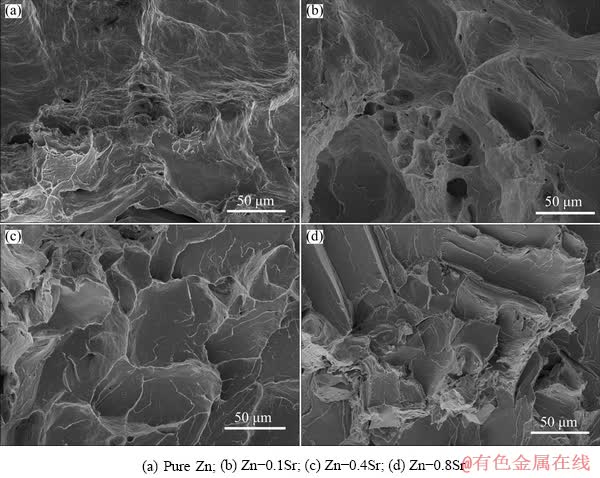
Fig. 6 Fracture surface morphologies of as-extruded pure Zn and Zn-xSr (x=0.1, 0.4, 0.8 wt.%) alloys

Fig. 7 Corrosion rates of as-extruded pure Zn and Zn-xSr (x=0.1, 0.4, 0.8 wt.%) alloys immersed in SBF solution at (37±0.5) °C for 480 h
Figure 7 shows the corrosion rates of as-extruded pure Zn and Zn-Sr binary alloys after immersion in SBF solution at (37±0.5) °C for 480 h. The corrosion rate of pure Zn was (11.45±2.02) μm/a, which was lower than that of Zn-Sr binary alloys. It was (26.59±3.33) μm/a for Zn-0.1Sr alloy, (30.47±2.57) μm/a for Zn-0.4Sr alloy and (32.59±3.40) μm/a for Zn-0.8Sr alloy. Hence, with the addition of 0.1 wt.% Sr into pure Zn, the corrosion rate was increased obviously, and further increase of the Sr content led to the slight increase of the corrosion rate. Figure 8 showed the surface morphologies and EDS results of degradation products. With increasing the Sr content, the degradation products tended to cluster together. Furthermore, all of those degradation products consisted of C, O, P, Ca and Zn, in a round sphere shape, demonstrating the possible existence of Zn carbonates or phosphates. The surface morphologies of the as-extruded pure Zn and Zn-xSr alloys after immersion in SBF solution at (37±0.5) °C for 480 h and then acid washing were exhibited in Fig. 9. Uniform surface morphologies were observed in pure Zn and Zn-0.1Sr alloys, while some small corrosion pits near the SrZn13 particles appeared in Zn-0.4Sr and Zn-0.8Sr alloys, indicating that the interface between Zn matrix and SrZn13 particles was corroded faster than matrix. Hence, with the addition of Sr over 0.1 wt.%, the corrosion rate increased and in vitro degradation behavior gradually changed from uniform corrosion into localized corrosion mode.
4 Discussion
4.1 Effect of Sr addition on microstructure

Fig. 8 Surface morphologies and EDS results of degradation products
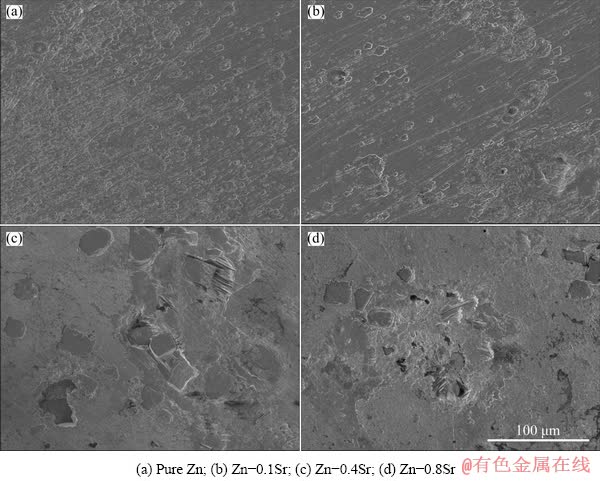
Fig. 9 Surface morphologies of as-extruded pure Zn and Zn-xSr alloys after immersion in SBF solution at (37±0.5) °C for 480 h and then acid washing
The SrZn13 phase was newly formed due to the addition of Sr, which was confirmed by XRD and EDS results. OM images indicated that the average size of SrZn13 phase was more than 30 μm. According to the Zn-Sr binary phase diagram [26], the melting point for pure Zn was about 419 °C and the solubility of Sr in Zn (liquid) was more than 1 wt.% at 600 °C, while the solubility of Sr in Zn (solid) was almost zero. In this study, those alloys were cast at 600 °C, with temperature cooling down from 600 to 419 °C provided enough time for the nucleation and growth of SrZn13 phase. The higher the Sr content is, the larger the size of SrZn13 phase will be. LIU et al [27] also confirmed that a slight increase of Sr content led to the dramatic rise of average size of SrZn13 phase. Therefore, in this study, with the increase of Sr content, the average size of SrZn13 phase increased gradually.
Actually, high stress zones would form around the coarse second phase during deformation, which promoted the dynamic recrystallization process, i.e., the PSN during recrystallization [25]. Unlike the dispersive nanoscale phase, the hard second phase particles with a diameter larger than about 1 μm could stimulate the nucleation of recrystallization significantly in the deformation zones near the particles. The size of hard particles played an important role in the PSN of recrystallization, which was confirmed in Al-7.9Zn-2.7Mg-2.0Cu alloy [28]. The dislocations could accumulate around the coarse particles during low temperature deformation, and deformation zones formed near the coarse particles after deformation would contribute to the refined microstructure. In this study, hard and coarse SrZn13 phases with the diameter of nearly 30 μm also served as nucleation sites to promote the recrystallization and retard the motion of dislocations. With the increase of Sr content, more and more SrZn13 phases would form, which generated more nucleation sites and enhanced the resistance to the motion of dislocations during the deformation. As a result, the grain would be refined due to the PSN effect.
With the addition of 1 wt.% Sr into Zn, the microhardness of the as-cast pure Zn significantly increased from HV 38.24 to HV 61.88 [16], increased by about 62%, which could be attributed to the formation of hard SrZn13 phase. In this study, SrZn13 phases were hardly broken into small pieces during hot extrusion, also implying that the hardness of Zn-1.0Sr alloy was much higher than that of Zn matrix under hot extrusion conditions. In addition, hard particles could hinder the migration of grain boundary [29]. Hence, the growth of dynamic recrystallization grains could be restrained by these hard SrZn13 particles. Therefore, grains nearby SrZn13 phase were smaller, and the average grain size after hot extrusion decreased with the increasing addition of Sr.
4.2 Effect of Sr addition on mechanical properties
According to the phase diagram of the Zn-Sr binary alloy [26], only a small amount of Sr could dissolve into Zn matrix, while the other Sr would form hard SrZn13 particles. Both of these two factors would affect the mechanical properties of Zn-Sr alloys. The former provided solid solution strengthening which would contribute to the tensile strength, while the precipitation of hard SrZn13 particles refined the grains of the as-extruded alloys. Grain refinement would further strengthen the Zn-Sr alloys compared with pure Zn. However, further increase of Sr content from 0.1 to 0.8 wt.% would lead to the decrease of strength regardless of the slightly refined grain size, which might be related to different sizes of SrZn13 particles in those alloys. Since the hardness of the SrZn13 particles was much higher than that of Zn matrix, during room temperature tensile test, deformation would be activated in Zn matrix firstly by dislocation gliding. When dislocations glide into the interface between the SrZn13 particles and matrix, they would pile-up. Since the amount of SrZn13 particles increased with the increase of Sr content, more area would appear to hinder the motion of dislocations. Thus, in Zn-0.4Sr and Zn-0.8Sr alloys, dislocations would pile-up more quickly around larger SrZn13 particles than the Zn-0.1Sr alloy reinforced with smaller SrZn13 particles. Rapid accumulation of dislocations would lead to the stress concentration at the sharp edge of the SrZn13 phase. Then, cracks might initiate and propagate in the soft Zn matrix near the hard SrZn13 phase, leading to premature failure. The similar phenomenon was observed in Zn-3Cu-1Fe alloy due to the formation of FeZn13 phase [17] and Al-Si-Mg alloy due to the formation of Fe-rich phase as impurity [30]. In this study, large sharp-edged SrZn13 phase formed when the Sr content was over 0.1 wt.%. Hence, for the Zn-0.4Sr and Zn-0.8Sr alloys, the cracks would be easier to form during deformation, resulting in the decrease of the mechanical properties. Therefore, the mechanical properties of Zn-Sr binary alloys were not simply in a positive relation to the increase of Sr, and Zn-0.1Sr alloy exhibited the best mechanical properties in this study.
4.3 Effect of Sr addition on degradation behavior
The standard electrode potential of Zn in aqueous solution is -0.762 V [10], and according to the Pourbaix diagram [1], the degradation behavior of matrix Zn in the SBF solution during the immersion test could be demonstrated by the following reaction:
2Zn+2H2O+O2=2Zn2++4OH- (2)
For pure Zn after immersion test, the corrosion rate was (11.45±2.02) μm/a, which was lower than that of Zn-Sr binary alloys. The degradation products of pure Zn exhibited uniform distribution. After the addition of Sr into the alloys, the corrosion rate was greatly improved by more than 185%. However, when the Sr content was increased further, the corrosion rate tended to increase slowly. The significant difference of corrosion rate between the pure Zn and Zn-Sr binary alloys could be explained by the formation of SrZn13 phase. The main role of the second phase played in the degradation behavior was to promote the micro galvanic corrosion, which was reported by many previous research works [31-33]. LIU et al [27] found that SrZn13 phase acted as an active intermediate with low equilibrium potential and resulted in galvanic corrosion. In this study, the formation of SrZn13 phase should also stimulate the galvanic corrosion and increase the corrosion rate. On the other hand, without this second phase, pure Zn exhibited uniform degradation behavior with plenty of degradation products covered on the samples surface. These degradation products served as a protective layer to prevent the transportation of O2 and H2O between the samples surface and the SBF solution, thus hindering further corrosion into the deep area of the samples. OM images in Figs. 9(c) and (d) demonstrated the corrosion area almost around the sharp edge of SrZn13 phases. With the increase of Sr content, more pits formed after immersion in SBF solution. Moreover, unlike the morphology of degradation products remaining on pure Zn, that of Zn-Sr binary alloys tended to cluster together, leaving the rest areas exposed directly to SBF solution. The inhomogeneous distribution of degradation products reduced the protective effect on the matrix, and enhanced the corrosion into deep pits.
5 Conclusions
(1) SrZn13 phase with sharp edges was newly formed and precipitated non-uniformly in the as-cast Zn-Sr binary alloys. The alloys recrystallized completely, but the shape and size of SrZn13 phase have no significant changes after hot extrusion due to its high hardness. Meanwhile, grains near the SrZn13 phase showed smaller size attributed to the PSN effect of SrZn13 particles.
(2) The mechanical properties including strength and elongation of Zn were enhanced by the addition of 0.1 wt.% Sr, and gradually decreased after further addition of Sr due to the precipitation of large-sized SrZn13 particles.
(3) With the increase of Sr, the corrosion rate increased and in vitro degradation behavior gradually changed from uniform corrosion into localized corrosion mode due to the galvanic corrosion between SrZn13 particles and Zn matrix.
References
[1] MOSTAED E, SIKORA-JASINSKA M, DRELICH J W, VEDANI M. Zinc-based alloys for degradable vascular stent applications [J]. Acta Biomaterialia, 2018, 71: 1-23.
[2] BOWEN P K, SHEAIER E R, ZHAO S, GUILLORY R J, ZHAO F, GOLDMAN J, DRELICH J W. Biodegradable metals for cardiovascular stents: From clinical concerns to recent Zn-alloys [J]. Advanced Healthcare Materials, 2016, 5: 1121-1140.
[3] HERMAWAN H. Updates on the research and development of absorbable metals for biomedical applications [J]. Progress in Biomaterials, 2018, 7: 93-110.
[4] SHEAIER E R, BOWEN P K, HE W L, DRENCH A, DRELICH J, GOLDMAN J, ZHAO F. In vitro cytotoxicity, adhesion, and proliferation of human vascular cells exposed to zinc [J]. ACS Biomaterials Science & Engineering, 2016, 2: 634-642.
[5] KATARIVAS LEVY G, GOLDMAN J, AGHION E. The prospects of zinc as a structural material for biodegradable implants—A review paper [J]. Metals, 2017, 7: 402-419.
[6] XIAO S J, WANG M, WANG L P, ZHU Y C. Environment- friendly synthesis of trace element Zn, Sr, and F codoping hydroxyapatite with non-cytotoxicity and improved osteoblast proliferation and differentiation [J]. Biological Trace Element Research, 2018, 185: 148-161.
[7] PRASAD A S. Zinc: A miracle element. Its discovery and impact on human health [J]. JSM Clinical Oncology and Research, 2014, 2: 1030-1036.
[8] BAFARO E, LIU Y, XU Y, DEMPSKI R E. The emerging role of zinc transporters in cellular homeostasis and cancer [J]. Signal Transduction Targeted Therapy, 2017, 2: e17029.
[9] BOWEN P K, DRELICH J, GOLDMAN J. Zinc exhibits ideal physiological corrosion behavior for bioabsorbable stents [J]. Advanced Materials, 2013, 25: 2577-2582.
[10] ANTELMAN M S, HARRIS F J. The encyclopedia of chemical electrode potentials [M]. Boston: Springer, 1982.
[11] JIN H L, ZHAO S, GUILLORY R, BOWEN P K, YIN Z Y, GRIEBEL A, SCHAFFER J, EARLEY E J, GOLDMAN J, DRELICH J W. Novel high-strength, low-alloys Zn-Mg (<0.1 wt.% Mg) and their arterial biodegradation [J]. Materials Science and Engineering C, 2018, 84: 67-79.
[12] VOJTECH D, KUBASEK J, SERAK J, NOVAK P. Mechanical and corrosion properties of newly developed biodegradable Zn-based alloys for bone fixation [J]. Acta Biomaterialia, 2011, 7: 3515-3522.
[13] LIU X W, SUN J K, QIU K J, YANG Y H, PU Z J, LI L, ZHENG Y F. Effects of alloying elements (Ca and Sr) on microstructure, mechanical property and invitro corrosion behavior of biodegradable Zn-1.5Mg alloy [J]. Journal of Alloys and Compounds, 2016, 664: 444-452.
[14] SOTOUDEH BAGHA P, KHALEGHPANAH S, SHEIBANI S, KHAKBIZ M, ZAKERI A. Characterization of nanostructured biodegradable Zn-Mn alloy synthesized by mechanical alloying [J]. Journal of Alloys and Compounds, 2018, 735: 1319-1372.
[15] NIU J L, TANG Z B, HUANG H, PEI J, ZHANG H, YUAN G Y, DING W J. Research on a Zn-Cu alloy as a biodegradable material for potential vascular stents application [J]. Materials Science and Engineering C, 2016, 69: 407-413.
[16] LI H F, XIE X H, ZHENG Y F, CONG Y, ZHOU F Y, QIU K J, WANG X, CHEN S H, HUANG L, TIAN L, QIN L. Development of biodegradable Zn-1X binary alloys with nutrient alloying elements Mg, Ca and Sr [J]. Scientific Reports, 2015, 5: 10719.
[17] YUE R, HUANG H, KE G Z, ZHANG H, PEI J, XUE G H, YUAN G Y. Microstructure, mechanical properties and in vitro degradation behavior of novel Zn-Cu-Fe alloys [J]. Materials Characterization, 2017, 134: 114-122.
[18] SHI Z Z, YU J, LIU X F. Microalloyed Zn-Mn alloys: From extremely brittle to extraordinarily ductile at room temperature [J]. Materials & Design, 2018, 144: 343-352.
[19] SHI Z Z, YU J, LIU X F, WANG L N. Fabrication and characterization of novel biodegradable Zn-Mn-Cu alloys [J]. Journal of Materials Science & Technology, 2018, 34: 1008-1015.
[20] PEMMER B, ROSCHGER A, WASTL A, HOFSTAETTER J G, WOBRAUSCHEK P, SIMON R, THALER H W, ROSCHGER P, KLAUSHOFER K, STRELI C. Spatial distribution of the trace elements zinc, strontium and lead in human bone tissue [J]. Bone, 2013, 57: 184-193.
[21] MARIE P J, AMMANN P, BOIVIN G, REY C. Mechanisms of action and therapeutic potential of strontium in bone [J]. Calcified Tissue International, 2014, 69: 121-129.
[22] DAHL S G, ALLAIN P, MARIE P J, MAURAAS Y, BOIVIN G, AMMANN P, TSOUDEROS Y, DELMAS P D, CHRISTIANSEN C. Incorporation and distribution of strontium in bone [J]. Bone, 2001, 28: 446-453.
[23] LIU F, CHEN X, CHEN B, ZHU Y R, YU L P. Microstructure and properties of Zn-xSr biodegradable medical materials [J]. Special Casting & Nonferrous Alloys, 2018, 38: 923-926.
[24] ASTM G31—72. Practice for laboratory immersion corrosion testing of metals [S]. Philadelphia, PA, USA, 2004.
[25] HUMPHREYS F J, MILLER W S, DJAZEB M R. Microstructural development during thermomechanical processing of particulate metal-matrix composites [J]. Materials Science and Technology, 1990, 6: 1157-1166.
[26] EDWARD J, KOTTCAMP H, LANGER E L. Alloy phase diagrams [M]. 3rd ed. Philadelphia: ASM Alloy Phase Diagram and Handbook Committees, 1992.
[27] LIU Y, GENG C, ZHU Y K, CHEN X. Effect of Sr addition on microstructure evolution and mechanical properties of Zn-4%Al hypoeutectic alloy [J]. Journal of Alloys and Compounds, 2017, 695: 443-451.
[28] ZANG Q, YU H, LEE Y S, KIM M S, KIM H W. Effects of initial microstructure on hot deformation behavior of Al-7.9Zn-2.7Mg-2.0Cu (wt%) alloy [J]. Materials Characterization, 2019, 151: 404-413.
[29] NIKULIN L, KIPELOVA A, MALOPHEYEV S, KAIBYSHEV R. Effect of second phase particles on grain refinement during equal-channel angular pressing of an Al-Mg-Mn alloy [J]. Acta Materialia, 2012, 60: 487-497.
[30] EISAABADI B G, DAVAMI P, KIM S K, VARAHRAM N, YOON Y O, YEOM G Y. Effect of oxide films, inclusions and Fe on reproducibility of tensile properties in cast Al-Si-Mg alloys: Statistical and image analysis [J]. Materials Science and Engineering A, 2012, 558: 134-143.
[31] GUESSOUM K, VEYS-RENAUX D, ROCCA E, BELHAMEL K. Corrosion behaviour of zinc–cerium alloys: Role of intermetallic phases [J]. Corrosion Science, 2011, 53: 1639-1645.
[32] SONG Y W, HAN E H, SHAN D Y, YIM C D, YOU B S. The effect of Zn concentration on the corrosion behavior of Mg-xZn alloys [J]. Corrosion Science, 2012, 65: 322-330.
[33] WANG Y Q, KONG G, CHEN C S. Corrosion behavior of Zn-Al alloys in saturated Ca(OH)2 solution [J]. Corrosion Science, 2016, 112: 679-686.
柯贵州1,岳 锐1,黄 华1,2,康 斌3,曾 晖3,袁广银1,2
1. 上海交通大学 材料科学与工程学院,轻合金精密成型国家工程中心和金属基复合材料国家重点实验室,上海 200240;
2. 上海创新材料研究所,上海 200444;
3. 北京大学深圳医院 骨科,深圳 518036
摘 要:系统研究纯锌和 Zn-xSr (x=0.1%, 0.4%, 0.8%, 质量分数)合金热挤压后的显微组织、力学性能及体外降解行为。研究发现:添加 0.1% Sr 后合金中析出SrZn13相,使挤压后合金的屈服强度、抗拉强度及伸长率由纯锌的(85.33±2.86) MPa、(106.00±1.41) MPa和(15.37±0.57)%分别提高到(107.67±2.05) MPa、(115.67±2.52) MPa和(20.80±2.19)%;继续增加Sr含量,由于析出粗大的SrZn13相,容易引用应力集中和裂纹产生,使合金的性能下降。此外,分布不均匀的SrZn13相与基体之间存在的微电偶腐蚀作用使得添加Sr后合金的体外降解速度加快,同时,降解变得不均匀。随着Sr含量的增加,降解速度逐渐加快,由纯锌的(11.45±2.02) mm/a逐渐增加至 Zn-0.8Sr的(32.59±3.40) mm/a。其中,挤压态Zn-0.1Sr 合金具有最佳的综合力学性能与降解性能。
关键词:锌锶合金;挤压;组织;力学性能;体外降解行为
(Edited by Wei-ping CHEN)
Foundation item: Project (17XD1402100) supported by the Science and Technology Commission of Shanghai Municipality, China; Project (SZSM201612092) supported by Shenzhen Three Renowned Project, China; Project (2018RC001A-18) supported by the Innovation Talent Program of Karamay City, China; Project (2018D01A07) supported by the Natural Science Foundation of Xinjiang Uygur Autonomous Region, China
Corresponding author: Hua HUANG; Tel: +86-21-34203051; Fax: +86-21-34202794; E-mail: huangh@sjtu.edu.cn
DOI: 10.1016/S1003-6326(20)65346-8

