钒渣钙镁复合焙烧-浸出提钒
来源期刊:中国有色金属学报(英文版)2020年第11期
论文作者:向俊一 王鑫 裴贵尚 黄青云 吕学伟
文章页码:3114 - 3123
关键词:钒;钒渣;焙烧;酸浸;回收率
Key words:vanadium; vanadium slag; roasting; acid leaching; recovery
摘 要:为了提高转炉钒渣提钒回收率,提出钙镁复合焙烧-酸浸提钒新工艺,研究MgO/(CaO+MgO)摩尔比、焙烧和浸出工艺参数对钒回收率的影响。结果表明:当焙烧添加剂CaO完全被MgO取代时,钒浸出率降低,由88%降至81%;然而,采用CaO/MgO复合焙烧却能强化钒的浸出。当MgO/(CaO+MgO)的摩尔比为0.5:1时,钒浸出率达到94%。XRD和SEM-EDS结果表明,CaO/MgO复合焙烧添加剂能强化焙烧过程中可溶性钒酸盐的生成,并通过减少硫酸钙沉淀的生成改善浸出过程的动力学条件。
Abstract: A novel process of composite roasting with CaO/MgO and subsequent acid leaching was proposed to improve the recovery rate of vanadium from Linz–Donawiz (LD) converter vanadium slag. The effects of the MgO/(CaO+MgO) molar ratio and the roasting and leaching parameters on the recovery of vanadium were studied. The results showed that the leaching efficiency of vanadium decreased from 88% to 81% when CaO was replaced completely by MgO; however, it could be improved by roasting with the composite of CaO/MgO. The maximum vanadium leaching efficiency of 94% was achieved under the optimum MgO/(CaO+MgO) mole ratio of 0.5:1. The results from X-ray diffractometry (XRD) and scanning electron microscopy with energy-dispersive X-ray spectroscopy (SEM-EDS) confirm that the formation rate of acid-soluble vanadates can be enhanced during roasting with the composite of CaO/MgO and that the leaching kinetics can be accelerated owing to the suppression of calcium sulfate precipitation.
Trans. Nonferrous Met. Soc. China 30(2020) 3114-3123
Jun-yi XIANG1, Xin WANG1, Gui-shang PEI1, Qing-yun HUANG2, Xue-wei Lü1,3
1. College of Materials Science and Engineering, Chongqing University, Chongqing 400044, China;
2. College of Metallurgy and Materials Engineering, Chongqing University of Science and Technology, Chongqing 401331, China;
3. Chongqing Key Laboratory of Vanadium-Titanium Metallurgy and Advanced Materials, Chongqing University, Chongqing 400044, China
Received 15 March 2020; accepted 29 July 2020
Abstract: A novel process of composite roasting with CaO/MgO and subsequent acid leaching was proposed to improve the recovery rate of vanadium from Linz–Donawiz (LD) converter vanadium slag. The effects of the MgO/(CaO+MgO) molar ratio and the roasting and leaching parameters on the recovery of vanadium were studied. The results showed that the leaching efficiency of vanadium decreased from 88% to 81% when CaO was replaced completely by MgO; however, it could be improved by roasting with the composite of CaO/MgO. The maximum vanadium leaching efficiency of 94% was achieved under the optimum MgO/(CaO+MgO) mole ratio of 0.5:1. The results from X-ray diffractometry (XRD) and scanning electron microscopy with energy-dispersive X-ray spectroscopy (SEM-EDS) confirm that the formation rate of acid-soluble vanadates can be enhanced during roasting with the composite of CaO/MgO and that the leaching kinetics can be accelerated owing to the suppression of calcium sulfate precipitation.
Key words: vanadium; vanadium slag; roasting; acid leaching; recovery
1 Introduction
Vanadium is widely used in various industries, including steel, chemistry, aerospace, and medicine, due to its excellent physicochemical properties [1,2]. Vanadium and its compounds originate mostly from primary sources such as ore feedstock and concentrates [3], secondary sources such as metallurgical slags, and petroleum residues [4]. Among these, vanadium-bearing slag is the most important source for vanadium, especially in China, where it accounts for approximately 85% of the vanadium production [5,6].
The steps in the process of extracting vanadium from vanadium slag include roasting, leaching, purification of the aqueous solution, and precipitation [7,8], of which roasting plays the most crucial role in the whole process chain. The objective of roasting is to convert the insoluble vanadium oxide into water-soluble sodium vanadate salts [9]. The primary and commercial approaches that are currently used to extract vanadium from vanadium slag are sodium salt roasting–water leaching [10,11] and calcification roasting–acid leaching [12]. Although sodium salt roasting is a mature process and a vanadium recovery rate of approximately 90% can be achieved [13], problems such as large-scale emission of corrosive gases (HCl, Cl2, SO2, and SO3) [14], easy slag fusion and agglomeration, and poor adaptability of raw materials still exist [15]. As opposed to sodium salt roasting, there are no hazardous gases and toxic tailings discharged during the calcification roasting process [16]. It shows obvious advantages in terms of energy, environments, and mineral resource utilization efficiency [17-19]. Several studies were conducted based on the calcification roasting process, including the effects of roasting additives [20], roasting parameters [21], leaching parameters [22], and the recovery efficiency of the vanadium [23]. Unfortunately, the recovery rate of vanadium components in the calcification roasting process was apparently lower than that of the sodium salt roasting process. In recent decades, many new processes have been performed to recover vanadium from vanadium-bearing converter slag, such as manganese salt roasting–acid leaching [24], microwave roasting [12], acid leaching by electro-oxidation [14], and liquid- oxidation [25]. However, only a few of these processes are applied to industrial production owing to economic reasons.
Roasting is a vital step in the vanadium recovery process. Magnesium and calcium are alkaline earth metals in the element periodic table, and follow similar rules in the phase transition during the high-temperature calcination process between the MgO-V2O5 and the CaO-V2O5 systems. The reactions between CaO, MgO, and V2O5 in the process of oxidation roasting are thermodynamically feasible, but the thermodynamic advantage of MgO with V2O5 is slightly weaker than that of CaO with V2O5 [26]. In the process of roasting, the formation of vanadate depends on the diffusion of ions in the solid phase. The radius of the magnesium ion is smaller than that of the calcium ion, resulting in a faster diffusion rate; therefore, the kinetics of the roasting process can be improved by adding MgO. As a result, the conversion efficiency of vanadium is potentially higher under the same roasting condition. In addition, for the leaching process, based on the characteristic that the solubility of magnesium sulfate is much higher than that of calcium sulfate, the precipitation of superfine calcium sulfate around the roasted product can be partially avoided in the leaching process. Therefore, a novel process to recover vanadium from vanadium slag, which included the composite roasting with CaO/MgO, and acid leaching, was proposed in the present study.
2 Experimental
2.1 Materials
Vanadium slag provided by Panzhihua Iron and Steel Group Corp (China) was used in this study. Chemical composition of the vanadium slag is mainly 15.29 wt.% V2O5, 14.38 wt.% TiO2, and the main phases are Fe2TiO4, (Mn,Fe)(V,Cr)2O4, and Fe2SiO4 [27]. The samples were crushed, ground, and sieved (with particle sizes <75 μm). The CaO and MgO were analytically pure, with purity greater than 99.9%. Deionized water was used in the experiment. The commercial sulfuric acid used was reagent grade with a concentration of 98% H2SO4 (Chongqing Chuandong Chemical Co., Ltd.). All the other reagents and chemicals used were of analytical reagent grade.
2.2 Procedure
A given amount of vanadium slag (20 g), and a certain amount of CaO and MgO powders were first mixed in a planetary ball mill (Retsch PM 100, Germany) with a rotation speed of 200 r/min. The milling was conducted in the air with a ball/mixture mass ratio of 10:1. The total operation time was 5 min, with a 1 min break after every 2 min of running.
In the roasting experiment, the milled samples were placed in alumina crucibles and roasted in a muffle furnace at 750-950 °C for 0.5-4.0 h under the oxidation atmosphere. The roasted compounds were characterized by X-ray diffractometry (XRD) and scanning electron microscopy (SEM) after cooling down to room temperature.
The leaching experiments were performed at atmospheric pressure. A 500 mL three-necked round-bottomed Pyrex flask equipped with a thermometer, a mechanical stirrer, and a reflux condenser was used as the batch reactor. The flask was heated by a thermostatically controlled water bath to reach and maintain the desired temperature within ±1 °C. A stirring speed of 200 r/min was adopted to keep the slurry suspended during the leaching experiment. After a reaction time of 60 min, the slurry was filtrated to obtain the leaching liquor and solid residues. The leaching liquor was diluted to 500 mL in a volumetric flask and chemically analyzed. The leaching residue was filtered and dried in an oven at 105 °C for 24 h. The leaching efficiency of vanadium was calculated by the following equation:
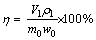 (1)
(1)
where η represents the leaching efficiency of vanadium (%), m0 is the mass of roasted slag (g), w0 is the mass fraction of vanadium in the roasted slag (%), V1 is the volume of leaching liquor (L), and ρ1 is the mass concentration of vanadium in the leaching liquor (g/L).
2.3 Characterization
The mineralogical compositions of vanadium slag and leaching residue were characterized by a powder X-ray diffractometer (PANalytical X’Pert Powder, PANalytical B. V.). The vanadium concentration of the solutions was analyzed by inductively coupled plasma optical emission spectrometry (ICP-OES) (ICAP 6000, Thermo Fisher Scientific, USA). An X-ray fluorescence (XRF) spectrometer (Swiss ARL Advant’ XP+-405) was employed to analyze the chemical composition of leaching residue. The SEM observation with the energy-dispersive X-ray spectroscopy (EDS) analysis of samples was performed by using the SEM (VEGA 3 LMH; TESCAN) equipped with an Oxford EDS.
3 Results and discussion
3.1 Roasting
3.1.1 MgO/(CaO+MgO) molar ratio
According to our experiments, the maximum vanadium recovery can be achieved by adding CaO or MgO with CaO/V2O5 or MgO/V2O5 in a molar ratio of 1.5:1. Therefore, the (CaO+MgO)/V2O5 molar ratio was maintained at 1.5:1 in all the experiments. The effect of MgO/(CaO+MgO) molar ratio was investigated with the ratio varying from 0 to 1:1 at a roasting temperature of 850 °C for 2 h. After roasting, the samples were ground into powders with the particle sizes <75 μm and then leached in sulfuric acid with a constant pH of 2.5 at leaching temperature of 50 °C, liquid/solid ratio of 20:1 (mL/g), and reaction time of 60 min.
Figure 1 shows the effect of MgO/(CaO+MgO) molar ratio on the leaching efficiency of vanadium. As evident from the results in Fig. 1, the leaching efficiency of vanadium increased from 87.85% to 93.80% as the molar ratios of MgO/(CaO+MgO) increased to 0.5:1, then sharply decreased to approximately 81% as MgO/(CaO+MgO) molar ratio continued to increase to 1:1. When only MgO was added to the slag, thermodynamic conditions were poor during roasting. When a composite of CaO and MgO was used instead, the thermodynamic and the kinetic conditions of the roasting process were improved. Based on these results, subsequent experiments were conducted with a MgO/(CaO+ MgO) molar ratio of 0.5:1.

Fig. 1 Effect of MgO/(CaO+MgO) molar ratio on leaching efficiency of vanadium
3.1.2 Roasting temperature
The effect of roasting temperature on the leaching of vanadium slag was investigated in the temperature range of 750 to 950 °C at a MgO/ (CaO+MgO) molar ratio of 0.5:1 and a fixed roasting time of 2 h. As shown in Fig. 2, it is clear that the leaching efficiency of vanadium significantly increased with increasing temperature, reached a maximum at 850 °C, and then dramatically decreased with the further increase in temperature. The roasting temperature is a key parameter for the extraction of vanadium. When the roasting temperature is lower than 850 °C, oxidation and conversion of vanadium become insufficient. Conversely, when the roasting temperature is too high, vanadium would be partially wrapped by glass phases that cannot be effectively leached in the sulfuric acid solution [27]. Therefore, the optimal roasting temperature was selected as 850 °C.
3.1.3 Roasting time
Roasting time is another important factor that influences vanadium leaching. Low-valent vanadium (V3+ and V4+) may not be completely oxidized to V5+ in a short roasting time. The effect of roasting time was be studied at 850 °C using five different roasting times (0.5, 1, 2, 3, and 4 h).
As shown in Fig. 3, the leaching efficiency of vanadium is greatly affected when the roasting time is less than 2 h. The leaching efficiency of vanadium reaches 54.36%, 70.72%, and 93.80% when roasted at 850 °C for 0.5, 1, and 2 h, respectively. However, no significant effect on the leaching efficiency of vanadium is observed with additional roasting time. Instead, the leaching efficiency of vanadium slightly decreases from 93.80% to 92.36% with an increase in the roasting time from 2 to 4 h. Generally speaking, long roasting time is beneficial to the solid-state reaction but leads to the fusion agglomeration of the samples. As a result, the formation of vanadate would be significantly controlled by the diffusion through the solid layer. Furthermore, the leaching of the vanadium can also be affected, because part of the vanadate is surrounded by the low-melting phase. For this reason, the subsequent experiments were conducted at 850 °C for 2 h.

Fig. 2 Effect of roasting temperature on leaching efficiency of vanadium
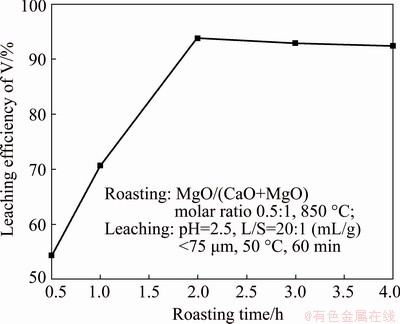
Fig. 3 Effect of roasting time on leaching efficiency of vanadium
3.2 Leaching
3.2.1 Leaching temperature
As mentioned above, the optimal roasting conditions occur at MgO/(CaO+MgO) molar ratio of 0.5:1, roasting temperature of 850 °C, and time of 2 h. The effect of leaching temperature on the leaching efficiency of vanadium was tested in the temperature range of 30 to 70 °C using a liquid/solid ratio of 20:1 (mL/g), and the results are shown in Fig. 4.
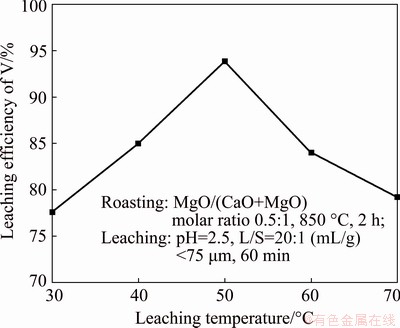
Fig. 4 Effect of leaching temperature on leaching efficiency of vanadium
As shown in Fig. 4, the leaching efficiency of vanadium is significantly affected by the leaching temperature, clearly increasing as the temperature is increased, reaching a maximum value of 93.80% at 50 °C, and then dramatically decreasing with the further increase in temperature. The high leaching temperature has a pronounced retarding effect on the reaction due to the hydrolysis of vanadium and difficulties in internal diffusion. Calcium has been reported to mainly precipitate as calcium sulfate and exist in the residue [28]. The production of CaSO4 could prevent vanadate from dissolving, resulting in a decline of the leaching efficiency of vanadium [29]. In fact, the ion diffusion resistance increases due to the encapsulation of calcium sulfate during leaching, resulting in internal diffusion-controlled leaching. Thus, a leaching temperature of 50 °C was selected as optimum temperature, and applied in the subsequent experiments.
3.2.2 Liquid/solid ratio
The effect of the liquid/solid ratio on the leaching of vanadium slag was examined using four liquid/solid ratios of 5:1, 10:1, 15:1, and 20:1 (mL/g), while other conditions were kept the same, as mentioned above. As expected, the results shown in Fig. 5 affirm that the leaching efficiency of vanadium is proportional to the liquid/solid ratio. As the liquid/solid ratio increases from 5:1 to 20:1 (mL/g), the leaching efficiency of vanadium rapidly increases from 73.63% to approximately 94%. As the amount of solution per unit mass of vanadium slag increases, the volume of filtrate increases, leading to a high leaching efficiency of vanadium. On the other hand, the kinetic rate of the multiphase reaction depends on the concentration difference of the reactants on both sides of the diffusion layer. The greater the concentration difference is, the faster the reaction rate is, and vice versa. Hence, as the liquid/solid ratio increases, the concentration difference increases, resulting in a better kinetic condition of ion diffusion. In addition, the leaching efficiency remains constant as the liquid/solid ratio increases from 20:1 to 25:1 (mL/g), so continuing to increase the liquid/solid ratio has little effect on the improvement of leaching efficiency. Therefore, the optimal liquid/solid ratio of 20:1 (mL/g) was selected.
Fig. 5 Effect of liquid/solid ratio on leaching efficiency of vanadium
3.2.3 Particle size
The effect of particle size on the leaching of vanadium slag was studied with the following four ranges: 150-300, 75-150, 50-75 and <50 μm, by using the optimum conditions mentioned above. The results given in Fig. 6 reveal that the leaching efficiency of vanadium is inversely proportional to the average initial diameter of the particles. Approximately 81.81% and 93.80% leaching efficiencies are achieved for the particle sizes of 150-300 μm and <50 μm, respectively. The specific surface area increases with the decrease in particle size, resulting in an increase of the reactant specific surface area between the slag particles and the H2SO4 solution, thus improving the reaction efficiency. Hence, the optimal particle size of <75 μm was selected taken into account economy and efficiency.
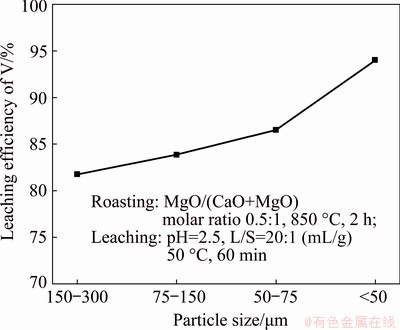
Fig. 6 Effect of particle size on leaching efficiency of vanadium
3.2.4 Acidity
The effect of acidity on the leaching of vanadium slag was performed using H2SO4, with the pH varying from 2.0 to 4.0, at a leaching temperature of 50 °C, liquid/solid ratio of 20:1 (mL/g), time of 60 min, and particle size smaller than 75 μm. The effect of acidity on the leaching behavior of vanadium is shown in Fig. 7.

Fig. 7 Effect of acidity on leaching efficiency of vanadium
As shown in Fig. 7, the leaching efficiency of vanadium is significantly increased from 36.95% at a pH of 2.0 to 93.80% at a pH of 2.5, then slightly decreased to 82.36% at a pH of 3.5, and finally markedly decreased to 62.81% at a pH of 4.0. The leaching efficiency of vanadium decreased with the increase in pH, which can be attributed to the low solubility of vanadium ions in a solution with low acidity [30], and the formation of a crystal nucleus of calcium sulfate [20]. Furthermore, the hydrolysis of vanadium occurred more easily when the pH was lower than 2.0, resulting in decreased leaching efficiency of vanadium at a pH of 2.0 [31]. In view of the above discussion, a moderate pH of 2.5 was chosen as the optimum leaching acidity in the current study.
3.3 Characterization of products
The XRD patterns of the vanadium slag roasted with different MgO/(CaO+MgO) molar ratios are shown in Fig. 8. The hematite (Fe2O3) and pseudobrookite (Fe2TiO5) are the major phases after roasting with different molar ratios of MgO/(CaO+ MgO). With the increase of MgO/(CaO+MgO) molar ratio to 1:1, the diffraction peaks of Ca2V2O7 gradually decreased, while those of Mg2V2O7 increased. However, the diffraction peaks of Mn2V2O7 slightly increased with increasing MgO/(CaO+MgO) molar ratios from zero to 0.5:1 and then decreased with higher MgO/(CaO+MgO) molar ratios. Furthermore, a new phase Ca5Mg4V6O24 is found in the roasted sample when the MgO/(CaO+MgO) molar ratio lies in the range of 0.17:1 to 0.5:1. Other vanadates, such as CaV2O6, MgV2O6, Ca3V2O8, and Mg3V2O8, are not found due to their low contents or poor crystallinity.
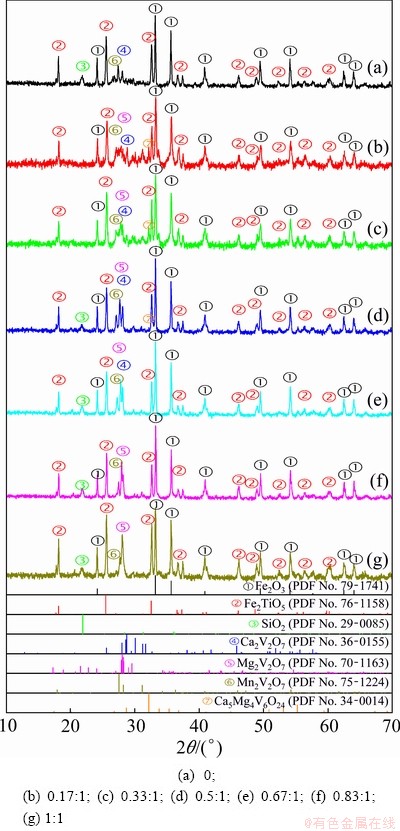
Fig. 8 XRD patterns of vanadium slag roasted with different MgO/(CaO+MgO) molar ratios
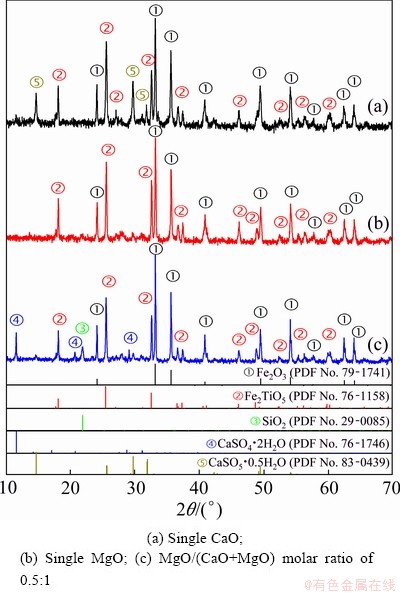
Fig. 9 XRD patterns of leaching residues: (a) Single CaO; (b) Single MgO; (c) MgO/(CaO+MgO) molar ratio of 0.5:1
The XRD patterns of the leaching residue are shown in Fig. 9. The major diffraction peaks of the leaching residue are still hematite (Fe2O3) and pseudobrookite (Fe2TiO5). However, the diffraction peaks of vanadates (Ca2V2O7, Mg2V2O7, Ca5Mg4V6O24, and Mn2V2O7) are not observed for the following reactions [19,24]:
Ca2V2O7+3H2SO4=(VO2)2SO4+2CaSO4+3H2O (2)
Mg2V2O7+3H2SO4=(VO2)2SO4+2MgSO4+3H2O (3)
Ca5Mg4V6O24+12H2SO4=3(VO2)2SO4+5CaSO4+4MgSO4+12H2O (4)
Mn2V2O7+2H2SO4=H4V2O7+2MnSO4 (5)
The diffraction peaks of hydrated calcium sulfate (CaSO4·0.5H2O) are found in the leaching residue of vanadium slag with the addition of single CaO (as shown in Fig. 9(a). Both CaSO4·0.5H2O and CaSO4·2H2O are detected in the leaching residue of vanadium slag with the addition of CaO and MgO (as shown in Fig. 9(c)). However, no diffraction peak of hydrated calcium sulfate is found in the leaching residue of vanadium slag with full MgO, as shown in Fig. 9(b).
The chemical compositions of the represented leaching residue are shown in Table 1. After leaching, the content of V2O5 in the leaching residue significantly decreased to 2.25%, 3.35%, and 0.52% for the samples roasted with CaO, MgO, and the composite with MgO/(CaO+MgO) molar ratio of 0.5:1, respectively. The content of S in the leaching residue significantly decreased from 3.64% to 0.06% for the sample roasted with MgO. This is attributed to the decrease in the formation of hydrated calcium sulfate. However, no obvious difference is observed in other components with different additives.
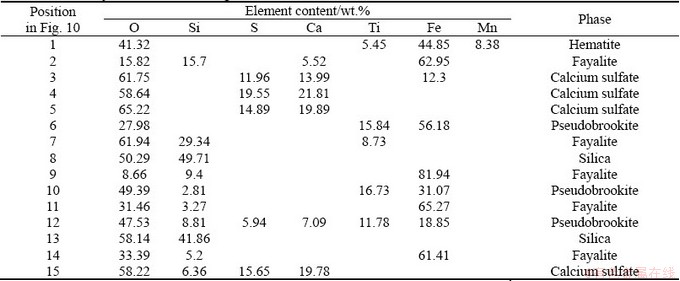
Further evidence of the mechanism involved during leaching of vanadium slag can be seen in Fig. 10 and Table 2, which depict SEM images and EDS analysis results, respectively, of the residue obtained after 60 min leaching under the optimum conditions mentioned above.
Different features are observed in the leaching residue with different additives. As shown in Figs. 10(a) and (b), there is a large number of rod-shaped and spherical particles produced in the leaching residue after adding single CaO. These particles can be identified as calcium sulfate, derived as an enrichment of O, Ca, and S at Positions 3, 4, 5, and 15 (as presented in Table 2). However, calcium sulfate is hardly produced when only MgO is added, as shown in Figs. 10(c) and (d). Small numbers of calcium sulfate particles (Position 15) can also be found in the leaching residue when adding the same molecular mass of CaO and MgO, as shown in Figs. 10(e) and 10(f). The main elements at Position 1 are Fe and O, indicating that Fe2O3 is the main phase in this position. The iron enrichment accompanied by Si and O at Positions 2, 7, 9, 11 and 14 can be identified as fayalite. The main elements at Positions 6, 10, and 12 are O, Fe, and Ti, which can be identified as pseudobrookite (Fe2TiO5). The main elements at Positions 8 and 13 are O and Si, thus revealing that the major phase is silica (SiO2). It is known that the calcium sulfate formed during the leaching process would be absorbed on the surface of vanadium slag and block the pore channel in the particles, which has a negative effect on the leaching process. However, the leaching efficiency of vanadium in vanadium slag with single MgO addition is much lower than that with single CaO. Therefore, there is strong evidence of the benefits of calcium-magnesium salt for the synergistic extraction of vanadium from the vanadium slag.
Table 1 Chemical compositions of leaching residue (wt.%)
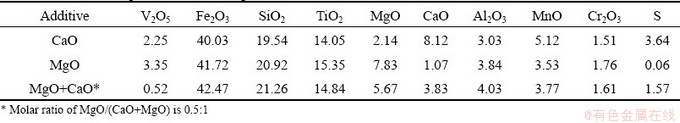

Fig. 10 SEM images of leaching residue with different additives
Table 2 EDS analysis results of leaching residue
4 Conclusions
(1) Full MgO roasting does not improve but worsens the recovery of vanadium. However, the leaching efficiency of vanadium increases with the addition of MgO to a composite of CaO and MgO, reaching a maximum at a MgO/(CaO+MgO) molar ratio of 0.5:1, then dramatically decreases with further addition of MgO.
(2) Both the roasting and leaching conditions have a significant influence on the leaching of vanadium slag. The leaching efficiency of vanadium can reach approximately 94% under the optimum roasting conditions of 850 °C, 2 h, MgO/(CaO+MgO) molar ratio of 0.5:1; and leaching conditions of 50 °C, liquid/solid ratio 20:1 (mL/g), particle size <75 μm, and a pH of 2.5.
(3) The XRD and SEM-EDS results confirmed that the formation rate of acid-soluble vanadates can be enhanced during roasting with the composite of CaO/MgO and that the leaching kinetics can be accelerated due to the suppression of calcium sulfate precipitation.
References
[1] VERNARDOU D, PATERAKIS P, DROSOS H, SPANAKIS E, POVEY I M, PEMBLE M E, KOUDOUMAS E, KATSARAKIS N. A study of the electrochemical performance of vanadium oxide thin films grown by atmospheric pressure chemical vapour deposition [J]. Solar Energy Materials & Solar Cells, 2011, 95(10): 2842-2847.
[2] DONG Ying-bo, LIU Yue, LIN Hai, LIU Chen-jing. Improving vanadium extraction from stone coal via combination of blank roasting and bioleaching by ARTP-mutated Bacillus mucilaginosus [J]. Transactions of Nonferrous Metals Society of China, 2019, 29(4): 849-858.
[3] LI Wang, ZHANG Yi-min, LIU Tao, HUANG Jing, WANG Yi. Comparison of ion exchange and solvent extraction in recovering vanadium from sulfuric acid leach solutions of stone coal [J]. Hydrometallurgy, 2013, 131: 1-7.
[4] PENG Xue-feng, ZHANG Yang, FAN Bing-qiang, ZHENG Shi-li, WANG Xiao-jian, ZHANG Ying, LI Ping, LIU Feng-qiang. Complexation separation for vanadium and chromium by dithiocarbamate and its application in treatment of chromium-vanadium-bearing slag [J]. Transactions of Nonferrous Metals Society of China, 2019, 29(11): 2400- 2410.
[5] MOSKALYK R R, ALFANTAZI A M. Processing of vanadium: A review [J]. Minerals Engineering, 2003, 16(9): 793-805.
[6] CHEN De-sheng, ZHAO Hong-xin, HU Guo-ping, QI Tao, YU Hong-dong, ZHANG Guo-zhi, WANG Li-na, WANG Wei-jing. An extraction process to recover vanadium from low-grade vanadium-bearing titanomagnetite [J]. Journal of Hazardous Materials, 2015, 294: 35-40.
[7] LOZANO L J, GODINEZ C. Comparative study of solvent extraction of vanadium from sulphate solutions by primene 81R and alamine 336 [J]. Minerals Engineering, 2003, 16(3): 291-294.
[8] CHEN De-sheng, ZHAO Long-sheng, LIU Ya-hui, QI Tao, WANG Jian-chong, WANG Li-na. A novel process for recovery of iron, titanium, and vanadium from titanomagnetite concentrates: NaOH molten salt roasting and water leaching processes [J]. Journal of Hazardous Materials, 2013, 244-245: 588-595.
[9] WANG Zhong-hang, ZHENG Shi-li, WANG Shao-na, LIU Biao, WANG Da-wei, DU Hao, ZHANG Yi. Research and prospect on extraction of vanadium from vanadium slag by liquid oxidation technologies [J]. Transactions of Nonferrous Metals Society of China, 2014, 24(5): 1273-1288.
[10] LI Xin-sheng, XIE Bing, WANG Guang-en, LI Xiao-jun. Oxidation process of low-grade vanadium slag in presence of Na2CO3 [J]. Transactions of Nonferrous Metals Society of China, 2011, 21(8): 1860-1867.
[11] CHEN Tie-jun, ZHANG Yi-min, SONG Shao-xian. Improved extraction of vanadium from a Chinese vanadium- bearing stone coal using a modified roast-leach process [J]. Asia-Pacific Journal of Chemical Engineering, 2010, 5(5): 778-784.
[12] GAO Hui-yang, JIANG Tao, ZHOU Mi, WEN Jing, LI Xi, WANG Ying, XUE Xiang-xin. Effect of microwave irradiation and conventional calcification roasting with calcium hydroxide on the extraction of vanadium and chromium from high-chromium vanadium slag [J]. International Journal of Mineral Processing, 2020, 145: 1-12.
[13] LI Xin-sheng, XIE Bing. Extraction of vanadium from high calcium vanadium slag using direct roasting and soda leaching [J]. International Journal of Minerals Metallurgy and Materials, 2012, 19(7): 595-601.
[14] LIU Zuo-hua, LI Yan, CHEN Man-li. NUERAIHEMAITI A, DU Jun, FAN Xing, TAO Chang-yuan. Enhanced leaching of vanadium slag in acidic solution by electro-oxidation [J]. Hydrometallurgy, 2016, 159: 1-5.
[15] HUANG J, ZHANG Y M, HUANG J, LIU T, CAI Z L, XUE N N. Selective leaching of vanadium from roasted stone coal by dilute sulfuric acid dephosphorization-two-stage pressure acid leaching [J]. Minerals, 2016, 6(3): 75-86.
[16] LI Hong-yi, WANG Kang, HUA Wei-hao, YANG Zhao, ZHOU Wang, XIE Bing. Selective leaching of vanadium in calcification-roasted vanadium slag by ammonium carbonate [J]. Hydrometallurgy, 2016, 160: 18-25.
[17] FU Zi-bi. Development process and trends of vanadium extraction from vanadium-titanium magnetite ore [J]. China Nonferrous Metallurgy, 2011, 40(6): 29-33. (in Chinese)
[18] CHEN Hou-sheng. Study on the extraction technology of V2O5 by calcination of lime from vanadium slag [J]. Iron Steel Vanadium Titanium, 1992(6): 3-11. (in Chinese)
[19] KOZLOV V A, DEMIDOV A E. Chemical principles of a technology for making pure vanadium pentoxide [J]. Metallurgist, 2000, 44(8): 428-433.
[20] PENG Hao, GUO Jing, ZHENG Xiao-gang, LIU Zuo-hua, TAO Chang-yuan. Leaching kinetics of vanadium from calcification roasting converter vanadium slag in acidic medium [J]. Journal of Environmental Chemical Engineering, 2018, 6(4): 5119-5124.
[21] ZHANG Ju-hua, ZHANG Wei, ZHANG Li, GU Song-qing. Mechanism of vanadium slag roasting with calcium oxide [J]. International Journal of Mineral Processing, 2015, 138: 20-29.
[22] WEN Jing, JIANG Tao, ZHOU Mi, GAO Hui-yang, LIU Jia-yi, XUE Xiang-xin. Roasting and leaching behaviors of vanadium and chromium in calcification roasting–acid leaching of high-chromium vanadium slag [J]. International Journal of Minerals Metallurgy and Materials, 2018, 25(5): 515-526.
[23] LI Lan-jie, ZHANG Li, ZHENG Shi-li, LOU Tai-ping, ZHANG Yi, CHEN Dong-hui, ZHANG Yan. Acid leaching of calcined vanadium titanomagnetite with calcium compounds for extraction of vanadium [J]. The Chinese Journal of Process Engineering, 2011, 11(4): 573-578. (in Chinese)
[24] WEN Jing, JIANG Tao, WANG Jun-peng, GAO Hui-yang, LU Long-gang. An efficient utilization of high chromium vanadium slag: Extraction of vanadium based on manganese carbonate roasting and detoxification processing of chromium-containing tailings [J]. Journal of Hazardous Materials, 2019, 378: 120733.
[25] WU Kang-hua, WANG Ya-ru, WANG Xin-ran, WANG Shao-na, LIU Biao, ZHANG Yi, DU Hao. Co-extraction of vanadium and chromium from high chromium containing vanadium slag by low-pressure liquid phase oxidation method [J]. Journal of Cleaner Production, 2018, 203: 873-884.
[26] JUNG I H, CAO Z M, QIAO Z Y, WANG N, XIE W. Critical evaluation and thermodynamic assessment of the MgO-V2O5 and CaO-V2O5 systems in air [J]. Calphad, 2017, 56: 72-79.
[27] XIANG Jun-yi, HUANG Qing-yun, LV Xue-wei, BAI Chen-guang. Effect of mechanical activation treatment on the recovery of vanadium from converter slag [J]. Metallurgical and Materials Transactions B: Process Metallurgy and Materials Processing Science, 2017, 48(5): 2759-2767.
[28] AARABI-KARASGANI M, RASHCHI F, MOSTOUFI N, VAHIDI E. Leaching of vanadium from LD converter slag using sulfuric acid [J]. Hydrometallurgy, 2010, 102: 14-21.
[29] YANG Zhao, LI Hong-yi, YIN Xu-chen, YAN Zhi-ming, YAN Xiao-man, XIE Bing. Leaching kinetics of calcification roasted vanadium slag with high CaO content by sulfuric acid [J]. International Journal of Mineral Processing, 2014, 133: 105-111.
[30] ZHOU Xue-jiao, WEI Chang, LI Min-ting, QIU Shuang, LI Xing-bin. Thermodynamics of vanadium-sulfur-water systems at 298 K [J]. Hydrometallurgy, 2011, 106(1-2): 104-112.
[31] GREENWOOD N N, EARNSHAW A. Chemistry of the elements [M]. 2nd ed. Oxford: Butterworth-Heinemann, 1997: 976-1001.
向俊一1,王 鑫1,裴贵尚1,黄青云2,吕学伟1,3
1. 重庆大学 材料科学与工程学院,重庆 400044;
2. 重庆科技学院 冶金与材料学院,重庆 401331;
3. 重庆大学 钒钛冶金及新材料重庆市重点实验室,重庆 400044
摘 要:为了提高转炉钒渣提钒回收率,提出钙镁复合焙烧-酸浸提钒新工艺,研究MgO/(CaO+MgO)摩尔比、焙烧和浸出工艺参数对钒回收率的影响。结果表明:当焙烧添加剂CaO完全被MgO取代时,钒浸出率降低,由88%降至81%;然而,采用CaO/MgO复合焙烧却能强化钒的浸出。当MgO/(CaO+MgO)的摩尔比为0.5:1时,钒浸出率达到94%。XRD和SEM-EDS结果表明,CaO/MgO复合焙烧添加剂能强化焙烧过程中可溶性钒酸盐的生成,并通过减少硫酸钙沉淀的生成改善浸出过程的动力学条件。
关键词:钒;钒渣;焙烧;酸浸;回收率
(Edited by Wei-ping CHEN)
Foundation item: Project (2018M640898) supported by the China Postdoctoral Science Foundation; Project (cstc2019jcyj-bshX0068) supported by the Natural Science Foundation of Chongqing, China; Project (52004044) supported by the National Natural Science Foundation of China; Project (2018YFC1900500) supported by the National Key Research and Development Program of China
Corresponding author: Jun-yi XIANG, E-mail: xiangjunyi126@126.com; Xue-wei Lü, E-mail: lvxuewei@163.com
DOI: 10.1016/S1003-6326(20)65447-4

