DOI: 10.11817/j.ysxb.1004.0609.2021-37885
轻稀土离子水合物Re3+(H2O)n (n=1~12,Re=La、Ce、Pr、Nd)的DFT研究
欧家才1, 2,张天锡1, 3,黄李金鸿4,吴伯增5,黄万抚1
(1. 江西理工大学 资源与环境工程学院,赣州 341000;
2. 五矿稀土集团广西五鑫矿业投资有限公司,南宁 530022;
3. 潍坊龙达锌业有限公司,潍坊 262100;
4. 江西理工大学 建筑与设计学院,赣州 341000;
5. 中国矿业大学(北京) 化学与环境工程学院,北京 100083)
摘 要:采用Material studio的DMol3模块研究了La3+、Ce3+、Pr3+、Nd3+四种离子的水合物Re3+(H2O)n (n=1~12),分析了几何优化后的构型的几何结构、结合能、前线轨道、电荷、振动情况。结果表明:La、Ce、Pr、Nd四种轻稀土离子Re3+的第一水化层,最小水合数分别为4、6、7、7;相同水合数条件下,Re—O平均键长:La>Ce>Pr>Nd,Re3+(H2O)10的Re—O平均键长约为2.6 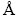 ,轻稀土离子水合物的水化层半径约为3.22~3.59
,轻稀土离子水合物的水化层半径约为3.22~3.59 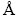 。轻稀土离子的水化反应为放热反应,当水分子配位数相同时,结合能大小顺序为:La>Ce>Pr>Nd。电荷分析结果显示,水合反应过程中稀土离子得到电子,当第一水化层填满后,Re3+(H2O)n性质逐渐趋于稳定,Re离子的电荷量在2e左右。前线轨道分析结果显示,不同于离子半径的变化,四种稀土离子自身的硬度顺序为:La>Nd>P>Ce。模拟了La3+(H2O)n的红外光谱,随着水分子数量的增加,Vsym和Vasym峰出现蓝移,当n大于10时,Vsym和Vasym峰不再发生蓝移,说明第一水化层外的水分子没有与La离子发生反应。
。轻稀土离子的水化反应为放热反应,当水分子配位数相同时,结合能大小顺序为:La>Ce>Pr>Nd。电荷分析结果显示,水合反应过程中稀土离子得到电子,当第一水化层填满后,Re3+(H2O)n性质逐渐趋于稳定,Re离子的电荷量在2e左右。前线轨道分析结果显示,不同于离子半径的变化,四种稀土离子自身的硬度顺序为:La>Nd>P>Ce。模拟了La3+(H2O)n的红外光谱,随着水分子数量的增加,Vsym和Vasym峰出现蓝移,当n大于10时,Vsym和Vasym峰不再发生蓝移,说明第一水化层外的水分子没有与La离子发生反应。
关键词:稀土;离子水合物;密度泛函;Material studio
文章编号:1004-0609(2021)-xx- - 中图分类号:TD955 文献标志码:A
引文格式:欧家才, 张天锡, 黄李金鸿, 等. 轻稀土离子水合物Re3+(H2O)n(n=1~12,Re=La、Ce、Pr、Nd)的DFT研究[J]. 中国有色金属学报, 2021, 31(x): xxxx-xxxx. DOI: 10.11817/j.ysxb.1004.0609.2021-37885
OU Jia-cai, ZHANG Tian-xi, HUANG Li-jin-hong, et al. DFT study on light rare earth ion hydrate Re3+(H2O)n (n=1~12, Re = La, Ce, Pr, Nd)[J]. The Chinese Journal of Nonferrous Metals, 2021, 31(x): xxxx-xxxx. DOI: 10.11817/j.ysxb.1004.0609.2021-37885
稀土金属被称为工业维生素,在催化、电磁、光学、农业和先进材料中有着重要和广泛的应用[1]。根据萃取分离时产生的不同质量分数的组别将稀土元素分为轻稀土、中稀土、重稀土三组:轻稀土元素(LRe)组包括镧到钕(原子序数从57到60);中稀土元素组(MRe)包括钐到钆(原子序数从62到64);重稀土元素(HRe)族由铽到镥(原子序数为65至71)和钇组成。
稀土离子在水溶液中会发生离子水化现象。离子水化现象在物理、化学、生物、地球化学和环境等领域引起广泛关注,其结果是离子进入水溶液后形成稳定的水合结构物,Ma+/b-(H2O)n[2-3]。该稳定的水合物是研究稀土离子在高岭石等黏土矿物表面吸附、解吸附(离子吸附型稀土矿浸出)、沉淀以及稀土萃取分离的重要基础。近年来,由于稀土元素的重要的化学相似性、特殊的电子性质和光学性质,三价稀土离子的水合结构物Re3+(H2O)n研究越来越受到重视,研究的手段主要包括计算机模拟,如分子动力学模拟[4-7]、量子化学模拟[6, 8-11]等,和光谱研究,如XRD[12-16]、EXAFS[16-18]、XANES[19]、Raman[20-22]等,以及热力学研究[23]等。现有的计算机模拟研究主要研究1-2中稀土离子的水化现象,没有将原子序数相近的稀土离子作为一个整体进行对比研究。光谱研究和热力学研究结果均显示Re3+与H2O的水合现象,但关于结合能的计算不够准确,容易受到外界条件的影响,如温度、水中其他离子等,此外,光谱研究对水合现象引起的光谱变化没有进行微观的解释,热力学研究对水合现象引起的结合能、电荷、轨道和光谱等信息联系起来共同解释稀土离子在水溶液中会发生离子水化现象。Re3+(H2O)n的形成过程受到水的极化性质影响,由于水分子配位数量的增加,水分子之间存在空间位阻效应:水分子数n较少时,(H2O)n与Re3+结合的更紧密;水分子数量n增加,水分子受到彼此的排斥作用,其配位键长逐渐增加,最终会形成一层致密的水分子层,即第一水化层;随着水分子数量的继续增加,Re原子周围开始形成第二甚至更多的水化层。从电子转移的角度分析,由于第一水化层距离中心的Re原子更近,与中心原子的电子转移更加突出。因此,本文将重点研究Re3+(H2O)n及其第一水化层的性质,在第一水化层的形成过程中,存在一个理论临界水分子数,当配位的水分子数超过这个临界值时,Re3+(H2O)n开始形成第二水化层。这个临界值并不是一成不变,因此,Re3+与H2O水化形成水合物是一个动态平衡过程,由于稀土离子外层丰富的电子数,自然条件下,其配位数介于9~12之间,其结果更容易在光谱和衍射的分析中出现。稀土离子的萃取分离遵循“先分组再分离”的策略,相同组的稀土离子具有相近似的性质但更不易分离,更需要了解同一组内不同稀土离子的不同水化行为及其微观解释,因此,本文主要目的是确定轻稀土组La、Ce、Pr和Nd三价离子的水合结构物的配位数(the coordination number,简称CN)及其在水溶液中的结构,建立起宏观的实验数据与微观结构的联系,达到更加精确地控制稀土离子浸出和分离[24]、稀土材料的制备等有关化学反应的进行。
1 计算参数
采用Material studio(MS)软件的DMol3模块进行稀土阳离子水合物构型优化,采用广义梯度近似(GGA)中的Perdew-Burke-Ernzerh(PBE)交换关联势描述电子交换相关作用。色散矫正采用DFT-D的TS修正。采用DSPP半核赝势,全电子(All electron)原子核处理方式,价电子波采用DNP展开。考虑到稀土元素的磁性,设置自旋极化。所有计算精度设置为Fine水平,受力、能量和位移收敛标准依次为0.54 eV/nm,2.72×10-5 eV和5×10-4 nm,截断半径0.44 nm。
2 计算结果
2.1 几何优化后的构型
水中的H: O—O的长度约为2.8 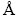 ,初始模型设置的稀土离子与水分子中氧原子的键长(简称Re—O键长)为2.8
,初始模型设置的稀土离子与水分子中氧原子的键长(简称Re—O键长)为2.8 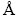 ,几何优化后,Re—O键长小于2.8
,几何优化后,Re—O键长小于2.8  ,则表示稀土离子与水分子相互吸引,具有成键的可能,反之则表示二者相互排斥。考虑稀土离子在成键时5d轨道和4f轨道的成键特性[25-28],几何优化完成后,允许软件自动重新计算所有轨道的成键情况(Monitor bonding)。图1~4为几何优化后稀土离子水合物的稳定构型,对该稳定构型进行了轨道能量计算并绘制LUMO轨道(Isosurface value=0.03a.u.),其中黄色部分为波函数为正的电子云,蓝色为波函数为负的电子云。表1为优化后的的键长结果,图5为根据优化结果绘制的稀土离子水合物的Re—O的键长图。
,则表示稀土离子与水分子相互吸引,具有成键的可能,反之则表示二者相互排斥。考虑稀土离子在成键时5d轨道和4f轨道的成键特性[25-28],几何优化完成后,允许软件自动重新计算所有轨道的成键情况(Monitor bonding)。图1~4为几何优化后稀土离子水合物的稳定构型,对该稳定构型进行了轨道能量计算并绘制LUMO轨道(Isosurface value=0.03a.u.),其中黄色部分为波函数为正的电子云,蓝色为波函数为负的电子云。表1为优化后的的键长结果,图5为根据优化结果绘制的稀土离子水合物的Re—O的键长图。
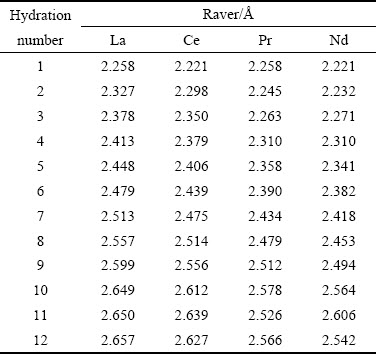
图1~4显示了四种稀土离子均可以结合1~10个水分子,形成水合结构物(以下简称水合物);当结合11个水分子时,La、Ce、Pr、Nd外围分别有1、3、2、1个水分子脱离;当结合12个水分子时,La、Ce、Pr、Nd外围均有2个水分子脱离。结合优化后的稳定构型可知,La、Ce、Pr、Nd第一水化层最大结合水个数为10。当配位数为10时,稀土与水分子中氧原子的平均键长约为2.6  ,表现出相似性。
,表现出相似性。
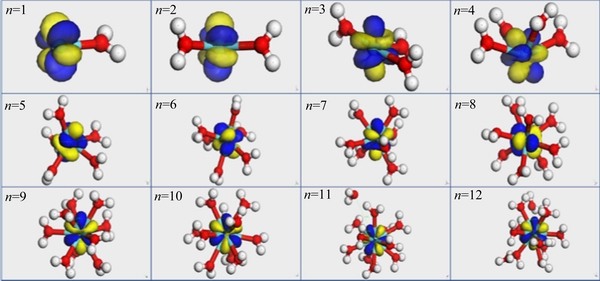
图1 几何优化后的 La3+(H2O)n(n=1~12)
Fig. 1 Geometry optimized La3+(H2O)n(n=1-12)
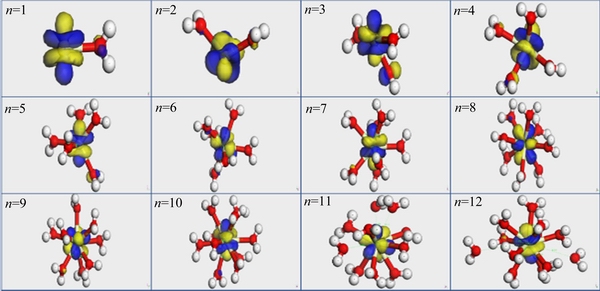
图2 几何优化后的Ce3+(H2O)n(n=1~12)
Fig. 2 Geometry optimized Ce3+(H2O)n(n=1-12)

图3 几何优化后的Pr3+(H2O)n(n=1~12)
Fig. 3 Geometry optimized Pr3+(H2O)n(n=1-12)
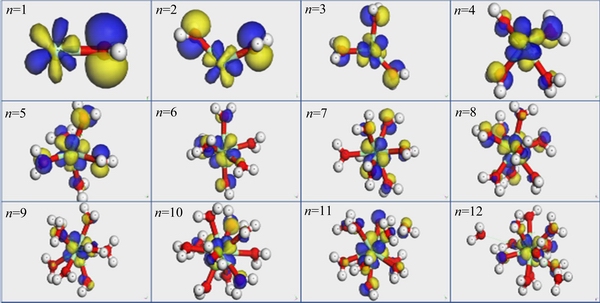
图4 几何优化后的Nd3+(H2O)n(n=1~12)
Fig. 4 Geometry optimized Nd3+(H2O)n(n=1-12)
表1 Re3+(H2O)n(n=1~12)中Re—O平均键长
Table 1 Average bond length of Re—O in Re3+(H2O)n (n=1-12)
理论上,水合物的水化层半径R计算公式如下
R=L(R—O)+L(O—H)*sinβ
式中:L(R—O)为中心稀土离子与水分子中O原子的键长,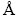 ;L(O—H)为水分子中O原子与H原子的键长,约为0.9
;L(O—H)为水分子中O原子与H原子的键长,约为0.9 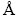
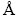 ;β为最远处水分子中O原子与H原子所成线段l与该键中O原子所在点处水化层切平面α的夹角,(°)。
;β为最远处水分子中O原子与H原子所成线段l与该键中O原子所在点处水化层切平面α的夹角,(°)。
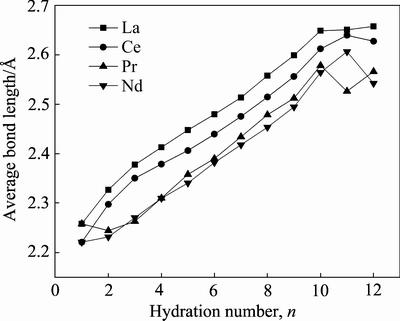
图5 Re3+(H2O)n(n=1~12)的Re—O的键长
Fig. 5 Re—O bond length of Re3+(H2O)n(n=1-12)
Re3+与H2O水化形成水合物是一个动态平衡过程,L(R—O)与β均是变化值。随着结合水分子数量的增加为10时,L(R—O)将逐渐稳定,约为2.6 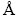 ;水分子的键角为104.5°,动态平衡过程,由于原子间的斥力,最远处水分子的H原子不会出现在切平面α之下,考虑到水分子的结构稳定性,β的取值范围应为37.75°~90°。因此,计算得到轻稀土离子水合物的水化层半径为3.22~3.59
;水分子的键角为104.5°,动态平衡过程,由于原子间的斥力,最远处水分子的H原子不会出现在切平面α之下,考虑到水分子的结构稳定性,β的取值范围应为37.75°~90°。因此,计算得到轻稀土离子水合物的水化层半径为3.22~3.59 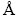 。
。
稀土元素常以6s、6p和5d轨道参与成键,其轨道总数为9,这是稀土化合物配位数在9左右的主要原因[25-28]。同时,如图5所示,当结合2~10个水分子时,Re—O键长的长度均为:La>Ce>Pr>Nd而离子半径值:La3+(106 pm)>Ce3+(103 pm)>Pr3+(101 pm)>Nd3+(100 pm),符合“金属离子的半径越大,其水化半径越大”的一般规律[9, 29-31]。
随着结合水分子数量的增加,稀土水合物中氧原子的平均键长逐渐增大,但仍然小于2.8 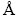 ,由此可见,在反应的过程中,稀土对溶液中的水分子具有一定的束缚能力,此外,众多的研究表明[32-35],水中的H: O—O的长度在2.8
,由此可见,在反应的过程中,稀土对溶液中的水分子具有一定的束缚能力,此外,众多的研究表明[32-35],水中的H: O—O的长度在2.8 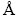 左右,水分子本身的O: H—O的长度大约在1.7
左右,水分子本身的O: H—O的长度大约在1.7 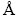 左右,四种稀土离子的Re—O键长介于两者之间,说明在水溶液中稀土与水分子的结合能力大于水分子之间的结合能力。
左右,四种稀土离子的Re—O键长介于两者之间,说明在水溶液中稀土与水分子的结合能力大于水分子之间的结合能力。
2.2 结合能
三价稀土离子与水分子之间形成稀土水合物的反应如下:
Re3++nH2O Re3+(H2O)n
Re3+(H2O)n
根据上述反应方程式,结合能(EB)按下式计算:
EB=EHydrates-ERe-nEH2O
为了研究相邻水合物种类转换的难度,根据以下反应式,计算了水合物种类间的相邻结合能(EAB):
Re3+(H2O)n+H2O Re3+(H2O)n+1
Re3+(H2O)n+1
EAB=EB(n+1)-EB(n)-EH2O
四种稀土离子与水的结合能计算结果如表2所示。
四种稀土离子与水结合的结合能均为负,说明水化过程为放热反应。随着结合水分子的数目增加,Re3+的EB值逐渐减小,与水分子的结合能力逐渐减弱。无论结合水数量为多少,结合能均为:La>Ce>Pr>Nd,与之前对比键长和离子半径得到的结合能力一致。
水分子团簇中氢键的键能在0.1772~0.2022 eV (0.0078~0.0089 Ha)之间[36],OH—H2O的键能约为0.9088 eV (0.040 Ha)[37]。四种稀土离子EAB的绝对值均大于氢键的键能,证明了稀土离子在与水结合时形成键的稳定性远大于氢键。当n=1~9时,四种稀土离子的EAB的绝对值均大于OH—H2O的键能,水分子更容易与稀土离子结合;当n超过9时,四种稀土离子的EAB的绝对值开始出现小于OH—H2O的键能的情况,在水溶液中会出现稀土离子与羟基争夺水分子的情况,这与几何优化后水合物出现水分子脱落的情形一致。
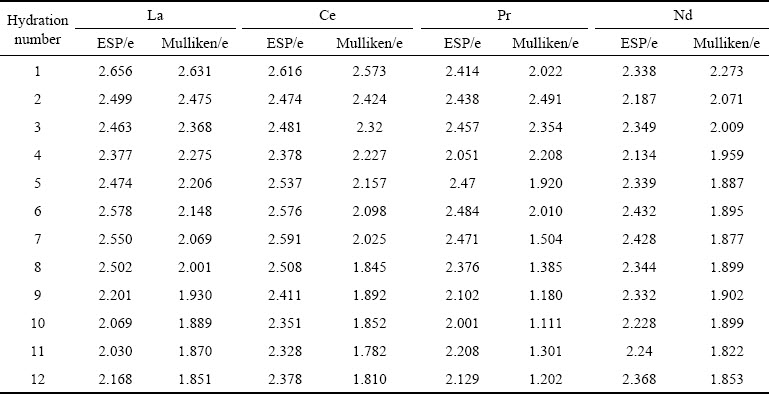
2.3 电荷分析
分别用ESP方法和Mulliken计算了Re3+(H2O)n中四种轻稀土金属的电荷分布情况,结果如表3所示。Re3+(H2O)n的形成过程是一个微弱的电荷转移过程。镧系三价金属离子在水化的过程中存在电子的转移,随着配位数的增加,Re原子从更多水分子中得到电子,Re原子电荷趋于减少,离子性降低,但是水合物的形成过程中电子转移的数量要小得多,通常小于1e,其结果就是Re离子在水溶液中仍然保持了正电性,其电荷量介于+2e到+3e之间。当n小于10时,随着n逐渐增加,Re离子的电荷数逐渐减小;当第一水化层填满后,Re离子的电荷数逐渐趋于稳定,此时的稀土离子水合物化学性质也逐渐稳定。
表2 Re3+(H2O)n(n=1~12)结合能EB与EAB
Table 2 Binding energy EB and EAB of Re3+(H2O)n(n=1-12)

表3 Re3+(H2O)n(n=1~12)中Re的电荷分布
Table 3 Charge distribution of Re in Re3+(H2O)n(n=1-12)
2.4 分子硬度和前线轨道分析
Re3+是Lewis硬酸[38-39],有非常强的亲氧性,与水分子的结合符合软硬酸碱理论中“硬亲硬”的结合原则[40]。LI,YAN和EVANS[41]证明了前线轨道理论可以理解为软硬酸碱(HSAB)原理的一部分。硬酸的特征是有低能量的空的价轨道—— LUMO轨道,硬碱的特征是具有可提供的低能量的价层HOMO电子对[40, 42]。
几何优化后稀土离子水合物的LUMO轨道(Isosurface value=0.03a.u.)显示在图1~4中。观察稀土水合物的LUMO轨道的外型可知,随着水分子数量的增加,稀土离子周围的LUMO轨道形状呈现出“阶段性变化”,以La3+(H2O)n为例,当n分别等于1、2;4、5、6和7;9、10、11和12时,LUMO轨道的外型相似。作为化学反应中最低的能量空轨道,LUMO轨道的改变说明体系最低能量的改变,进一步说明在结合水的过程中发生了电子的转移。当结合9个水分子以后,四种稀土离子的Re—O键长等仍然发生了微妙的变化并且可以在第一水化层或第二水化层继续结合水分子,但LUMO轨道的形状变化较小,说明此时的体系已经趋于稳定。与之相对应的,随着结合水的增加,稀土水合物的LUMO值逐渐变大,增长速度逐渐放缓,亦说明稀土元素的水合数逐渐饱和。四种稀土离子水合物的LUMO或HOMO轨道能量如表4所示。
使用物质的LUMO或HOMO轨道能量不足以完整的表示物质的硬度。因此,本文采用以下两种方式表示水合物的硬度:
1) 使用水合物自身的LUMO轨道能量值与HOMO轨道能量值的差来表示水合物自身的硬度:
△E=ELUMO-EHOMO
能量差越大,则硬度越大,酸碱的软硬通过酸碱形成的配位键中共价键的贡献得以体现[43]。
2) 使用水合物的LUMO轨道能量值与水分子的HOMO轨道能量值的差的绝对值来表示水合物相对于水分子的硬度:
|△E|=EHydrates/LUMO-EH2O/HOMO
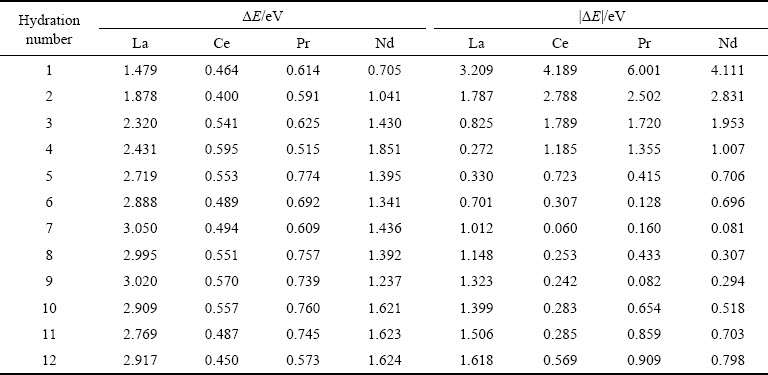
四种稀土离子水合物的|△E|与△E计算结果如表5所示。
表4 Re3+(H2O)n(n=1~12)中Re的HOMO轨道和LUMO轨道能量
Table 4 Energy calculation results of HOMO orbital and LUMO orbital of Re in Re3+(H2O)n(n=1-12)
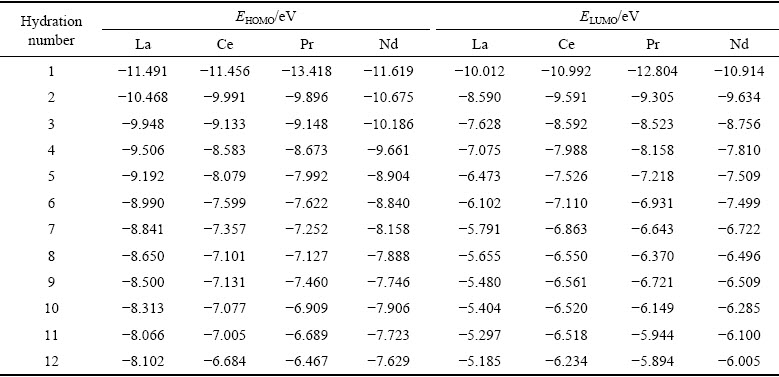
表5 Re3+(H2O)n(n=1~12)中Re的|△E|与△E计算结果
Table 5 Calculation results of |△E| and △E of Re in Re3+(H2O)n(n=1-12)
如图6所示,四种稀土离子自身的硬度顺序为:La>Nd>Pr>Ce。稀土元素失去电子的顺序为:最外层6s电子>次外层5d电子>倒数第三层4f电子。当形成三价金属阳离子时,稀土元素的最外层电子排列发生变化,理论的核外电子排布为:La3+:[Xe];Ce3+:[Xe]4f1;Pr3+:[Xe]4f2;Nd3+:[Xe]4f3。此时,La3+的理论HOMO轨道为5p轨道,Ce3+、Pr3+、Nd3+的理论HOMO轨道为4f轨道;La3+、Ce3+的理论LUMO轨道为5d轨道,Pr3+、Nd3+的理论LUMO轨道为6s轨道。根据Pauling近似能级图和Cotton原子轨道能级图可知,由于La3+的HOMO和LUMO轨道跨越了两个能级组,△E最大,硬度最高,Ce3+、Pr3+、Nd3+的HOMO和LUMO轨道同在一个能级组之内,△E值的差距主要受核外电子数影响。
如图7所示,分别为稀土离子水合物的LUMO值和水的HOMO的差值及其绝对值,在结合水的过程中,体系的△E值逐渐变大,当La、Ce、Pr、Nd分别结合4、6、7、7个水时,体系的的|△E|最靠近0,说明在水溶液环境中,Re3+的三价阳离子水合水个数为4、6、7、7时极易与H2O发生水合。从结合能的大小来看,当结合的H2O数量一致时,总是La与H2O的结合能最强,但此时,La可能结合的最小可能H2O数却最少,Ce、Pr、Nd可能结合的最小可能H2O数近似。分子轨道理论认为能量值之差的绝对值(|△E|)越小,两反应物之间的相互作用就越强,相同的环境下,水分子提供的HUMO轨道能量相同,但四种稀土离子具有不同的LUMO能量:La的LUMO能量值最高,Ce、Pr、Nd的LUMO能量值近似且低于La。所以,同为轻稀土组的四种稀土离子表现出不同的最小可能结合的水分子数。
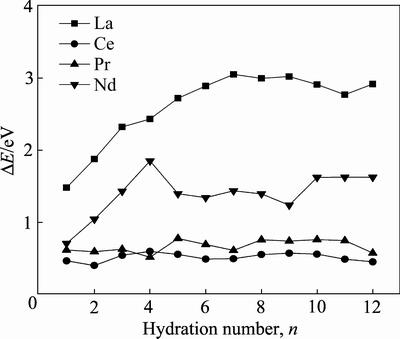
图6 Re3+(H2O)n(n=1~12)中Re离子△E变化
Fig. 6 Re ion △E in Re3+(H2O) n(n=1-12)〕
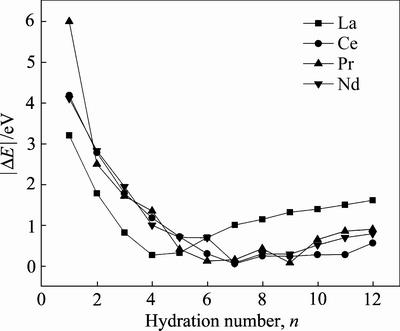
图7 Re3+(H2O)n(n=1~12)中Re离子|△E|变化
Fig.7 Re ion |△E| in Re3+(H2O)n(n=1-12)
2.5 振动分析
用PBE方法计算了Re3+(H2O)n簇的红外吸收光谱。由于在OH拉伸区域的测量方便,红外光谱可以为Re3+(H2O)n的研究提供详细的信息。图8所示为La3+(H2O)n(n=1~12)簇的红外光谱。La3+(H2O)n的对称拉伸振动(Vsym)峰位于约3546、3620、3640、3671、3687、3695、3698、3704、3704、3708、3708、3698 cm-1处,不对称拉伸振动(Vasym)峰位于约3629、3686、3733、3754、3764、3780、3789、3792、3791、3791、3807、3804 cm-1处。可以看出,n=1~6时,随着n的增加,Vsym和Vasym峰出现蓝移,这与增加电荷溶剂化和减少从金属离子到水分子的电荷转移是一致的, Zn2+(H2O)n[44]、Cd2+(H2O)n[44]、Hg2+(H2O)n[44]、Ni+(H2O)n[45]、K+(H2O)n[46]和Ca2+(H2O)n[47]水合物也观察到了这种蓝移。但是,如图8所示,当n=7~10时,Vsym和Vasym峰的蓝移逐渐减小,特别的,当n=11、12时,Vsym和Vasym峰基本不会蓝移,这与电荷分析情况一致。
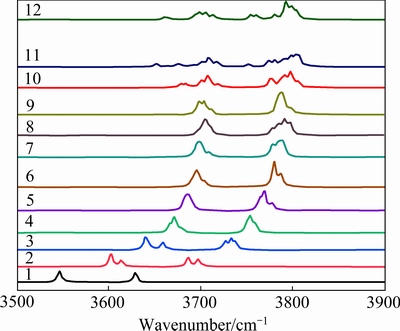
图8 模拟结构的La3+(H2O)n(n=1~12)的红外光谱
Fig. 8 Infrared spectrum of La3+(H2O)n(n=1-12) with simulated structure
3 结论
1) 轻稀土离子La3+、Ce3+、 Pr3+、Nd3+能够在水溶液中形成稳定的第一水化层,前线轨道分析结果显示,稳定的水合数n不小于4、6、7、7;第一水化层最多可以包含10个水分子。
2) 形成稳定的第一水化层后,轻稀土离子La3+、Ce3+、Pr3+、Nd3+的水合物Re3+(H2O)n的性质呈现出规律性和相似性。相同水合数条件下,Re—O平均键长:La>Ce>Pr>Nd,Re3+(H2O)10的Re—O平均键长约为2.6 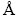 ,轻稀土离子水合物的水化层半径约为3.22~3.59
,轻稀土离子水合物的水化层半径约为3.22~3.59 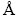 。轻稀土离子的水化反应均为放热反应,当水分子配位数相同时,结合能大小顺序为:La>Ce>Pr>Nd。电荷分析结果显示,Re离子的电荷量都在2e左右。前线轨道分析结果显示,四种稀土离子自身的硬度顺序为:La>Nd>Pr>Ce。
。轻稀土离子的水化反应均为放热反应,当水分子配位数相同时,结合能大小顺序为:La>Ce>Pr>Nd。电荷分析结果显示,Re离子的电荷量都在2e左右。前线轨道分析结果显示,四种稀土离子自身的硬度顺序为:La>Nd>Pr>Ce。
3) 第一水化层外的水分子与轻稀土离子La3+、Ce3+、 Pr3+、Nd3+的结合强度明显弱于第一水化层内的水分子。当n大于10时,Re—O键长明显更长,结合能变化减小,Re离子的电荷量变化明显减小,前线轨道能量值变化减小, La3+(H2O)n的红外光谱Vsym和Vasym峰不在发生蓝移。
REFERENCES
[1] LIM X. Chemistry: Degrees of separation[J]. Nature, 2015, 520(7548): 426-427.
[2] TEYCHENE J, BALMANN H R, MARON L, et al. Investigation of ions hydration using molecular modeling[J]. Journal of Molecular Liquids, 2019, 294: 111394.
[3] ABDELSALAM H, TELEB N H, YAHIA I S, et al. First principles study of the adsorption of hydrated heavy metals on graphene quantum dots[J]. Journal of Physics and Chemistry of Solids, 2019, 130: 32-40.
[4] CLAVAGUERA C, POLLET R, SOUDAN J M, et al. Molecular dynamics study of the hydration of lanthanum(Ⅲ) and europium(Ⅲ) including many-body effects[J]. The Senior Engineer Journal of Physical Chemistry B, 2005, 109(16): 7614-7616.
[5] SESSA F, SPEZIA R, D'ANGELO P. Lutetium(Ⅲ) aqua ion: On the dynamical structure of the heaviest lanthanoid hydration complex[J]. Journal of Chemical Physics, 2016, 144(20).
[6] TIRLER A O, PASSLER P P, RODE B M. The lanthanoid hydration properties beyond the ‘Gadolinium Break’: Dysprosium(Ⅲ) and holmium(Ⅲ), an ab initio quantum mechanical molecular dynamics study[J]. Chemical Physics Letters, 2015, 635: 120-126.
[7] MARJOLIN A, GOURLAOUEN C, CLAVAGUERA C, et al. Hydration Gibbs free energies of open and closed shell trivalent lanthanide and actinide cations from polarizable molecular dynamics[J]. Journal of Molecular Modeling, 2014, 20(10).
[8] ZHANG J, HEINZ N, DOLG M. Understanding Lanthanoid(Ⅲ) Hydration Structure and Kinetics by Insights from Energies and Wave functions[J]. Inorganic Chemistry, 2014, 53(14): 7700-7708.
[9] HOFER T S, WEISS A K H, RANDOLF B R, et al. Hydration of highly charged ions[J]. Chemical Physics Letters, 2011, 512(4): 139-145.
[10] FUJIWARA T, MORI H, MOCHIZUKI Y, et al. Theoretical study of hydration models of trivalent rare-earth ions using model core potentials[J]. Journal of Molecular Structure: Theochem, 2010, 949(1): 28-35.
[11] BUZKO V, SUKHNO I, BUZKO M. Ab initio and DFT study of Lu3+ hydration[J]. Journal of Molecular Structure: THEOCHEM, 2009, 894(1): 75-79.
[12] SMIRNOV P, KRITSKII I, GRECHIN O. Models of the nearest surrounding of ions in aqueous solutions of dysprosium chloride[J]. Russian Journal of Physical Chemistry A, 2016, 90(2): 406-410.
[13] HABENSCHUSS A, SPEDDING F H. The coordination (hydration) of rare earth ions in aqueous chloride solutions from X-ray diffraction. Ⅰ. TbCl3, DyCl3, ErCl3, TmCl3 and LuCl3[J]. The Journal of Chemical Physics, 2008, 70(6): 2797.
[14] HABENSCHUSS A, SPEDDING F H. The coordination (hydration) of rare earth ions in aqueous chloride solutions from X-ray diffraction. Ⅱ. LaCl3, PrCl3 and NdCl3[J]. The Journal of Chemical Physics, 2008, 70(8): 3758.
[15] HABENSCHUSS A, SPEDDING F H. The coordination (hydration) of rare earth ions in aqueous chloride solutions from X-ray diffraction. Ⅲ. SmCl3, EuCl3 and series behavior[J]. The Journal of Chemical Physics, 2008, 73(1): 442.
[16] NASLUND J, LINDQVIST-REIS P, PERSSON I, et al. Steric effects control the structure of the solvated lanthanum(Ⅲ) ion in aqueous, dimethyl sulfoxide, and N,N′-dimethylpropyleneurea solution. An EXAFS and large-angle X-ray scattering study[J]. Inorganic Chemistry, 2000, 39(18): 4006-4011.
[17] D'ANGELO P, ZITOLO A, MIGLIORATI V, et al. Revised ionic radii of lanthanoid(Ⅲ) ions in aqueous solution[J]. Inorganic Chemistry, 2011, 50(10): 4572-4579.
[18] PERSSON I, D'ANGELO P, De PANFILIS S, et al. Hydration of lanthanoid(Ⅲ) ions in aqueous solution and crystalline hydrates studied by EXAFS spectroscopy and crystallography: The myth of the “gadolinium break”[J]. Chemistry-A European Journal, 2008, 14(10): 3056-3066.
[19] D'ANGELO P, ZITOLO A, MIGLIORATI V, et al. Analysis of the detailed configuration of hydrated lanthanoid(Ⅲ) ions in aqueous solution and crystalline salts by using K- and L3-Edge XANES spectroscopy[J]. Chemistry-A European Journal, 2010, 16(2): 684-692.
[20] RUDOLPH W, IRMER G. On the hydration of heavy rare earth ions: Ho3+, Er3+, Tm3+, Yb3+ and Lu3+: A Raman study[J]. MOLECULES, 2019, 24(10).
[21] RUDOLPH W W, IRMER G. Raman spectroscopic characterization of light rare earth ions: La3+, Ce3+, Pr3+, Nd3+ and Sm3+ hydration and ion pair formation[J]. Dalton Transactions, 2017, 46(13): 4235-4244.
[22] RUDOLPH W, IRMER G. Hydration and ion pair formation in aqueous Lu3+ solution[J]. Molecules, 2018, 23(12): 3237.
[23] MARTELLI F, ABADIE S, SIMONIN J, et al. Lanthanoids(Ⅲ) and actinoids(Ⅲ) in water: Diffusion coefficients and hydration enthalpies from polarizable molecular dynamics simulations[J]. Pure and Applied Chemistry, 2012, 85(1): 237-246.
[24] YU D, DU R, XIAO J, et al. Theoretical Study of pKa values for trivalent rare-earth metal cations in aqueous solution[J]. The Journal of Physical Chemistry A, 2018, 122(2): 700-707.
[25] 李咏峰, 邝钜炽. 稀土的结构特点与4f轨道在成键中的作用[J]. 稀土, 2008(2): 100-101.
LI Yong-feng, KUANG Ju-chi. Structure feature of RE and effect of its 4f orbital in bonding process[J]. Chinese Rare Earths, 2008(2): 100-101.
[26] 张莉芳. 稀土4f5d电子成键特性及其在导电材料中结构与性质的调谐[D]. 合肥: 中国科学技术大学, 2019.
ZHANG Li-fang. Rare-earth 4f/5d electrons and their bonding characters in modulation both on structure and properties of conductive materials[D]. Hefei: University of Science and Technology of China, 2019.
[27] 赵 路. 基于稀土离子4f-4f 和4f-5d跃迁的光学温度传感[D]. 合肥:中国科学技术大学, 2019.
ZHAO Lu. Optical temperature sensing based on 4f-4f and 4f-5d transitions of rare earth ions[D]. Hefei: University of Science and Technology of China, 2019.
[28] 徐 慰. 镧系元素4f轨道在+2/+3价化合物中的成键性质[D]. 上海: 上海交通大学, 2015.
XU Wei. The bonding properties of lanthanide 4f orbits in di-/trivalent compounds[D]. Shanghai: Shanghai Jiao Tong University, 2015.
[29] SUN C Q, HUANG Y, ZHANG X. Hydration of Hofmeister ions[J]. Advances in Colloid and Interface Science, 2019, 268: 1-24.
[30] SOLDATOV V, ZELENKOVSKII V, KOSANDROVICH E. Hydration of ion exchangers: thermodynamics and quantum chemistry calculations. Ⅱ. An improved variant of the predominant hydrates model[J]. Reactive and Functional Polymers, 2016, 102: 147-155.
[31] SOLDATOV V, ZELENKOVSKII V, KOSANDROVICH E. Hydration of ion exchangers: Thermodynamics and quantum chemistry calculations. Ⅲ. The state of the proton and water molecules in hydrogen form of sulfostyrene ion exchangers[J]. Reactive and Functional Polymers, 2016, 102: 156-164.
[32] SIGALA P A, RUBEN E A, LIU C W, et al. Determination of hydrogen bond structure in water versus aprotic environments to test the relationship between length and stability[J]. Journal of the American Chemical Society, 2015, 137(17): 5730-5740.
[33] ZNAMENSKIY V S, GREEN M E. Quantum calculations on hydrogen bonds in certain water clusters show cooperative effects[J]. Journal of Chemical Theory and Computation, 2007, 3(1): 103-114.
[34] MOLLER K B, REY R, HYNES J T. Hydrogen bond dynamics in water and ultrafast infrared spectroscopy: A theoretical study[J]. The Journal of Physical Chemistry A, 2004, 108(7): 1275-1289.
[35] UMEYAMA H, MOROKUMA K. The origin of hydrogen bonding. An energy decomposition study[J]. Journal of the American Chemical Society, 1977, 99(5): 1316-1332.
[36] WENDLER K, THAR J, ZAHN S, et al. Estimating the hydrogen bond energy[J]. The Journal of Physical Chemistry A, 2010, 114(35): 9529-9536.
[37] MELTON C E. Formation of, by termolecular reactions, and bond dissociation energy, structure and bond length for OH-H2O and O-H2O[J]. The Journal of Physical Chemistry, 1972, 76(22): 3116-3120.
[38] 刘祁涛. 酸碱软硬度的键参数标度[J]. 化学通报, 1976(6): 26-32.
LIU Qi-tao. Key parameter scale of acid-base softness and hardness[J]. Chemistry, 1976(6): 26-32.
[39] 顾 均, 丁 祎, 柯 俊, 等. 基于软硬酸碱理论的单分散中重稀土硫氧化物纳米板的可控合成[J]. 化学学报, 2013, 71(3): 360-366.
GU Jun, DING Yi, KE Jun et al. Controllable synthesis of monodispersed middle and heavy rare earth oxysulfide nanoplates based on the principles of HSAB theory[J]. Acta Chimica Sinica, 2013, 71(3): 360-366.
[40] 沈文英, 张鲁勉, 何小英. 软硬酸碱规则的应用[J]. 汕头大学医学院学报, 2000(2): 17-19.
SHEN Wen-ying, ZHANG Lu-mian, HE Xiao-ying. Application of hard and soft acids and bases rule[J]. Journal of Shantou University Medical College, 2000(2): 17-19.
[41] LI Y, EVANS J N S. The Fukui function: A key concept linking frontier molecular orbital theory and the hard-soft-acid-base principle[J]. Journal of the American Chemical Society, 1995, 117(29): 7756-7759.
[42] 王纪镇, 印万忠. 酸碱软硬度的势标度在浮选剂结构性能研究中应用[J]. 东北大学学报(自然科学版), 2013, 34(7): 1035-1038.
WANG Ji-zhen, YIN Wan-zhong. Application of acid-base potential scale in structure performance study of flotation reagents[J]. Journal of Northeastern University(Natural Science), 2013, 34(7): 1035-1038.
[43] PEARSON R G. Recent advances in the concept of hard and soft acids and bases[J]. Journal of Chemical Education, 1987, 64(7): 561.
[44] YUAN X, ZHANG C. Density functional theory study on the inner shell of hydrated M2+(H2O)1-7 cluster ions for M=Zn, Cd and Hg[J]. Computational and Theoretical Chemistry, 2019: 112666.
[45] WALTERS R S, PILLAI E D, DUNCAN M A. Solvation dynamics in Ni+(H2O)n clusters probed with infrared spectroscopy[J]. Journal of the American Chemical Society, 2005, 127(47): 16599-16610.
[46] ZHU F, ZHOU H, ZHOU Y, et al. The investigation of structure and IR spectra for hydrated potassium ion clusters K+(H2O)n=1-16 by density functional theory[J]. The European Physical Journal D, 2016, 70(11).
[47] BUSH M F, SAYKALLY R J, WILLIAMS E R. Hydration of the calcium dication: Direct evidence for second shell formation from infrared spectroscopy[J]. Chem Phys Chem, 2007, 8(15): 2245-2253.
DFT study on light rare earth ion hydrate Re3+(H2O)n (n=1-12, Re = La, Ce, Pr, Nd)
OU Jia-cai1, 2, ZHANG Tian-xi1, 3, HUANG Li-jin-hong4, WU Bo-zeng5, HUANG Wan-fu1
(1. School of Resource and Environmental Engineering, Jiangxi University of Science and Technology. Ganzhou 341000, China;
2. Guangxi Wuxin Mining Investment, Subsidiary of China Minmetals Rare Earth Group Co., Ltd., Nanning 530022, China;
3. WeifangLongda Zinc Industry Co. LTD., Weifang 262100, China;
4. School of Architecture and Design, Jiangxi University of Science and Technology, Ganzhou 341000, China;
5. School of Chemical and Environmental Engineering, China University of Mining & Technology, Beijing 100083, China)
Abstract: DMol3 module of Material Studio was used to study the hydrate Re3+(H2O)n(n=1-12) of four light rare earth ion coordination water quantities of La, Ce, Pr and Nd. The geometric structure, binding energy, frontier molecular orbital, charge and vibration of the geometrically optimized configuration were analyzed. The results show that the minimum hydration number of the first hydration layer of La, Ce, Pr and Nd are 4, 6, 7 and 7 respectively, which can contain up to 10 water molecules. The average Re—o bond length of La>Ce>Pr>Nd, the average Re—O bond length of Re3+(H2O)10 was about 2.6 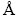 , the hydration layer width of light rare earth ionic hydrate was about 3.22-3.59
, the hydration layer width of light rare earth ionic hydrate was about 3.22-3.59 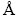 . The hydration reaction of light rare earth ions is exothermic. When the coordination number of water molecules is the same, the order of binding energy is La>Ce>Pr>Nd. Charge analysis results show that rare earth ions gain electrons in the hydration reaction. When the first hydration layer is filled, the properties of Re3+(H2O)n gradually become stable, and the charge of Re ions is around 2e. The results of frontier molecular orbital analysis show that, different from the change of ion radius, the hardness sequence of the four rare earth ions is La>Nd>Pr>Ce. The infrared spectrum of La3+(H2O)n was simulated. With the increase of the number of water molecules, the Vsym and Vasym peaks showed blue shift. When n was greater than 10, the Vsym and Vasym peaks did not show blue shift, indicating that the water molecules outside the first hydration layer did not react with La ions.
. The hydration reaction of light rare earth ions is exothermic. When the coordination number of water molecules is the same, the order of binding energy is La>Ce>Pr>Nd. Charge analysis results show that rare earth ions gain electrons in the hydration reaction. When the first hydration layer is filled, the properties of Re3+(H2O)n gradually become stable, and the charge of Re ions is around 2e. The results of frontier molecular orbital analysis show that, different from the change of ion radius, the hardness sequence of the four rare earth ions is La>Nd>Pr>Ce. The infrared spectrum of La3+(H2O)n was simulated. With the increase of the number of water molecules, the Vsym and Vasym peaks showed blue shift. When n was greater than 10, the Vsym and Vasym peaks did not show blue shift, indicating that the water molecules outside the first hydration layer did not react with La ions.
Key words: rare earth; ionic hydrate; density functional; Material studio
Foundation item: Project(41362003) supported by the National Natural Science Foundation of China
Received date: 2020-12-03; Accepted date: 2021-08-03
Corresponding author: HUANG Wan-fu; Tel: ; E-mail: Sim2008@sina.com
(编辑 )
基金项目:国家自然科学基金资助项目(41362003)
收稿日期:2020-12-03;修订日期:2021-08-03
通信作者:黄万抚,教授,博士;电话: ;E-mail:Sim2008@sina.com