文章编号:1004-0609(2014)12-3142-05
双氧水协同盐酸分解人造白钨
王小波,李江涛,张文娟,何贵香,赵中伟
(中南大学 冶金与环境学院,长沙 410083)
摘 要:利用双氧水易与钨形成可溶性过氧钨酸的特性,提出了双氧水(H2O2)协同盐酸分解人造白钨(CaWO4)的新工艺,使CaWO4以氯化钙(CaCl2)和过氧钨酸(H4[WO3(O2)2])的形式溶解到浸出体系中,大幅提高了人造白钨的分解率。研究盐酸和双氧水浓度、反应温度、液固比及反应时间等因素对人造白钨分解效果的影响。结果表明:在H2O2存在的情况下,盐酸用量可大幅降低,所用的盐酸初始浓度很低故挥发损失很少;H2O2浓度的增加有利于反应的进行;当温度低于30 ℃时,升高温度对人造白钨的分解浸出有明显的促进作用,超过40 ℃后H2O2会逐渐分解且产物过氧钨酸变得不稳定反而不利于浸出。得到的较优条件如下:H2O2浓度2.4 mol/L,盐酸浓度1.5 mol/L,温度30 ℃,液固比10:1(mL/g),反应时间40 min。在此条件下,人造白钨实现了完全分解。
关键词:人造白钨;盐酸;双氧水;过氧钨酸
中图分类号:TF841.1 文献标志码:A
Decomposition of synthetic scheelite in HCl solution with hydrogen peroxide as complexing agent
WANG Xiao-bo, LI Jiang-tao, ZHANG Wen-juan, HE Gui-xiang, ZHAO Zhong-wei
(School of Metallurgy and Environment, Central South University, Changsha 410083, China)
Abstract: A new process was proposed for the digestion of synthetic scheelite (CaWO4) in hydrochloric acid solution using hydrogen peroxide (H2O2) as complexing agent. H2O2 as a chelating agent, can readily react with H2WO4 to form water-soluble peroxotungstic acid H4[WO3(O2)2], and the conversion rate of WO3 was raised substantially. The effects of relevant parameters, such as the concentrations of HCl and H2O2, reaction temperature, liquid-to-solid ratio and reaction time, were studied. The results show that increasing the concentration of H2O2 benefits the process and both the concentrate and consumption of HCl drastically drop and the volatility loss of HCl reduces noticeably. Elevating the temperature promotes the decomposition of CaWO4 below 30 ℃ while becomes unfavorable above 40 ℃ because the stability of H2O2 and peroxotungstic acid gradually decreases. The optimum process conditions are as follows: the concentrations of H2O2 and HCl are 2.4 and 1.5 mol/L, respectively, the temperature is 30 ℃, the liquid-to-solid ratio is 10:1 (mL/g), and the reaction time is 40 min. Under these conditions the synthetic scheelite can be completely decomposed.
Key words: synthetic scheelite; hydrochloric acid; hydrogen peroxide; peroxotungstic acid
我国是钨资源大国,其资源储量、产量和出口量均居世界第一[1],资源储量中2/3以上为白钨资源。
目前,人造白钨(CaWO4)呈仅次于白钨矿和黑钨矿的第三类钨资源[2]的趋势,它主要来源于回收市场价值低廉的中低品位钨矿的碱浸出液[2-3]、含钨合金的边角料和废料[4-5]等,以及来源于低品位钨渣的资源化再生。
现阶段人造白钨的处理主要套用传统的天然白钨矿处理工艺。白钨矿的主要工艺为碱压煮工艺[6-8]和苏打压煮工艺[9]。由于该类反应的平衡常数不大,往往采用高温、高压和高浓度等强化手段来提高分解率,故能耗高、试剂消耗量大。传统的盐酸分解工艺[10]虽然反应平衡常数很大,具有可在常压下快速反应、流程短等优点,但是由于过程会产生胶态状黄钨酸,覆盖在未浸出的矿粒表面而阻碍反应的进行,因此不得不提高盐酸浓度和反应温度来促进反应的进行,而这势必会加剧盐酸的挥发和设备的腐蚀。自贡硬质合金厂通过采取加强密封[11]的措施,一定程度上改善了分解过程中的操作环境,但是进料、出料和过滤等操作均在敞开式环境中进行,盐酸的挥发难以避免。另外,为了消除固体钨酸的阻碍作用,瑞典可乐满企业曾采用机械球磨的方式进行边磨边浸,虽然大幅降低了盐酸浓度和反应温度,但是对球磨设备提出了更高的要求[10];此外,还有研究报道采用超声波[12-13]的方式来剥离钨酸膜,进而降低盐酸浓度和反应温度,改善浸出环境,但超声波设备的产业化仍需一段进程。
一种消除固体钨酸阻碍作用的思路是:在盐酸分解过程中,配合加入易与钨形成可溶性钨酸盐的络合剂,在本质上避免了固体膜的产生。常用的络合剂有磷酸、草酸、柠檬酸和酒石酸[14-16]等。考虑到双氧水分子中氧原子上的电子云密度大于磷酸和有机酸中配位氧原子的电子云密度,双氧水较之具有更强的络合能力;此外,双氧水价格相对低廉且不会带入外来杂质。因此,选用双氧水为络合剂,研究人造白钨在盐酸体系中的浸出行为,以期实现CaWO4在低盐酸浓度及低温下的高效分解。
1 实验
1.1 实验原料
实验所用原料为人造白钨,由Na2WO4和CaCl2按一定方法在实验室合成,经干燥和过筛,小于粒径0.074 mm粒级含量高于99%,其中,经辛可宁质量法测定WO3含量为78.67%。人造白钨的XRD谱如图1所示。从图1中可以看出,人造白钨接近纯相的钨酸钙。盐酸为浸出剂,双氧水为络合剂。实验中所有试剂都为分析纯。
1.2 实验原理
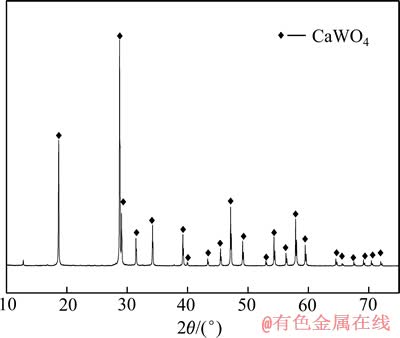
图1 人造白钨的XRD谱
Fig. 1 XRD pattern of synthetic scheelite
LASSNER等[17]认为,过氧根离子作为双原子配体,在取代钨酸根离子钨氧键上的氧原子时会形成一个三元环结构 ,当双氧水浓度达到一定值时,该结构在热力学上比
,当双氧水浓度达到一定值时,该结构在热力学上比 或
或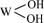 更稳定。因此,在双氧水存在的条件下,钨酸生成以后,不会产生固态沉淀,而是立即与双氧水中的过氧根离子进行络合反应形成可溶性的过氧钨酸。但是过氧钨酸并不稳定,随着n(
更稳定。因此,在双氧水存在的条件下,钨酸生成以后,不会产生固态沉淀,而是立即与双氧水中的过氧根离子进行络合反应形成可溶性的过氧钨酸。但是过氧钨酸并不稳定,随着n(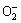 ):n(W)(过氧根与钨原子的摩尔比)比值的减小,其稳定性将会降低。随着酸度的变化,两者的比值也会发生变化,即存在不同物系之间的转化。DICKMAN等[18]报道了n(
):n(W)(过氧根与钨原子的摩尔比)比值的减小,其稳定性将会降低。随着酸度的变化,两者的比值也会发生变化,即存在不同物系之间的转化。DICKMAN等[18]报道了n(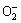 ):n(W)为4:1、3:1、2:1和1:1的实验研究。蒋安仁等[19]通过钨酸钠溶液制备钨酸的实验研究,认为只要H2O2与W的摩尔比大于1:2,即可达到络合钨的目的。
):n(W)为4:1、3:1、2:1和1:1的实验研究。蒋安仁等[19]通过钨酸钠溶液制备钨酸的实验研究,认为只要H2O2与W的摩尔比大于1:2,即可达到络合钨的目的。
溶液中的钨含量易于测量,过氧根离子的含量则通常采用高锰酸钾滴定法进行计算,由因此不难判断反应体系中可能存在的物系种类。通常采用n(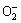 ):n(W)=2:1的物系表示过氧钨酸。因此,人造白钨的分解过程可以用如下反应方程式来表示:
):n(W)=2:1的物系表示过氧钨酸。因此,人造白钨的分解过程可以用如下反应方程式来表示:
CaWO4(s)+2HCl=H2WO4(s)+CaCl2(aq) (1)
H2WO4(s)+2H2O2=H4[WO3(O2)2](aq)+H2O (2)
总反应式为
CaWO4(s)+2H2O2+2HCl=H4[WO3(O2)2](aq)+CaCl2(aq)+H2O (3)
1.3 实验装置和操作
反应在250 mL三颈烧瓶中进行,中间用于放置搅拌装置,另两颈分别插置水银温度计和冷凝回流管。三颈烧瓶置于恒温水浴锅中,使实验保持在恒温条件下进行。实验装置图如图2所示。
每次实验称取10.00 g人造白钨,从三颈瓶的一颈均匀且缓慢地加入预热到设定温度的反应剂中,并开始计时。分解反应结束立即用布氏漏斗进行真空抽滤,并将滤液连同洗液进行定容,稀释到一定倍数后用ICP法测定其中的WO3浓度,并采用差减法计算出人造白钨的浸出率。

图2 双氧水协同盐酸分解人造白钨的实验装置
Fig. 2 Experimental apparatus of synthetic scheelite decomposition in HCl solution with hydrogen peroxide as complexing agent
2 结果与讨论
2.1 盐酸浓度对人造白钨浸出率的影响
在c(H2O2)=2.4 mol/L、液固比10:1、反应温度30 ℃和反应时间40 min的条件下,考察盐酸浓度对人造白钨浸出率的影响,实验结果如图3所示。
从图3可以看出,盐酸浓度对人造白钨的浸出影响很小。当盐酸浓度为0.8 mol/L(按理论用量c(HCl)为0.695 mol/L)时,WO3浸出率即超过95%;盐酸浓度增至2.5 mol/L时,WO3浸出率达到97.8%;超过2.5 mol/L时,随着盐酸浓度的增大,即体系酸度的提高,人造白钨的浸出率略有降低,这可能与产物过氧钨酸在高酸度下的稳定性下降有关。基于缓和对设备的腐蚀和减轻环境负担,最终确定最佳盐酸浓度为1.5 mol/L,其他因素实验都在该盐酸浓度下进行。
2.2 双氧水浓度对人造白钨浸出率的影响
在c(HCl)=1.5 mol/L、液固比10:1、反应温度30 ℃和反应时间40 min的条件下,考察双氧水浓度对人造白钨浸出率的影响,结果如图4所示。
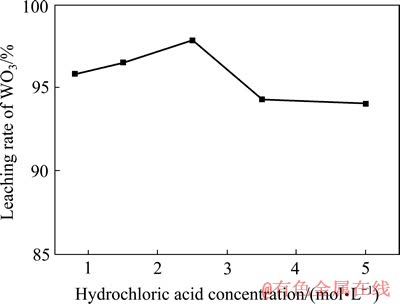
图3 盐酸浓度对人造白钨浸出率的影响
Fig. 3 Effect of hydrochloric acid concentration on leaching rate of WO3

图4 双氧水浓度对人造白钨浸出率的影响
Fig. 4 Effect of hydrogen peroxide concentration on leaching rate of WO3
由图4可知,随着双氧水浓度的不断提高,人造白钨的浸出率也逐步增大。当双氧水浓度达到2.4 mol/L时,人造白钨分解率达到97.5%以上,继续提高双氧水浓度WO3浸出率不再有明显变化。该实验双氧水浓度范围较大,n(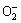 ):n(W)从略高于1:2(c(H2O2)= 0.2 mol/L)一直到大于4:1(c(H2O2)=1.6 mol/L)。当该比值略高于1:1时,WO3浸出率不到80%;该值高于4:1时,WO3浸出率超过90%;该比值继续增大,WO3浸出率则进一步提高。这说明双氧水浓度的提高不仅能加快反应速率,也有可能提高产物过氧钨酸在浸出体系中的稳定性,使过氧钨酸由n(
):n(W)从略高于1:2(c(H2O2)= 0.2 mol/L)一直到大于4:1(c(H2O2)=1.6 mol/L)。当该比值略高于1:1时,WO3浸出率不到80%;该值高于4:1时,WO3浸出率超过90%;该比值继续增大,WO3浸出率则进一步提高。这说明双氧水浓度的提高不仅能加快反应速率,也有可能提高产物过氧钨酸在浸出体系中的稳定性,使过氧钨酸由n(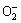 ):n(W)较小的物系向该比值较大的物系转变。最终选取双氧化浓度2.4 mol/L作为其较优浓度条件,其他因素实验保持该双氧水浓度不变。
):n(W)较小的物系向该比值较大的物系转变。最终选取双氧化浓度2.4 mol/L作为其较优浓度条件,其他因素实验保持该双氧水浓度不变。
2.3 反应温度对人造白钨浸出率的影响
在c(HCl)=1.5 mol/L、c(H2O2)=2.4 mol/L、液固比10:1和反应时间40 min的条件下,考察反应温度对人造白钨浸出率的影响,结果如图5所示。
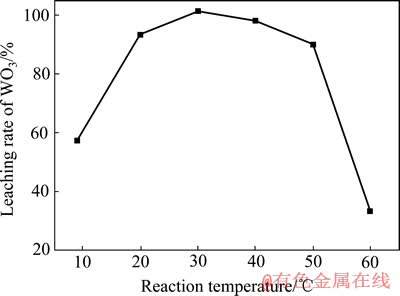
图5 反应温度对人造白钨浸出率的影响
Fig. 5 Effect of reaction temperature on leaching rate of WO3
结果表明:当温度较低时,升高温度对人造白钨的浸出有明显的促进作用,在上述实验条件下,温度达到30 ℃时WO3浸出率达到100%。而温度超过40 ℃以后,随着温度的继续升高,WO3浸出率下降的幅度越来越大。实际上只是水溶性的钨含量随温度升高明显降低,而人造白钨在之前的阶段经历了全部转化为水溶性过氧钨酸的过程,这一方面是由于双氧水在温度超过40 ℃后分解速率加快[20],另一方面过氧钨酸的稳定性随着温度的升高和体系双氧水浓度的降低而急剧下降所造成的。故确定实验的最佳温度为30 ℃,其他因素实验都在该温度下进行。
2.4 液固比对人造白钨浸出率的影响
在c(HCl)=1.5 mol/L、c(H2O2)=2.4 mol/L、反应温度30 ℃和反应时间40 min的条件下,考察液固比对人造白钨浸出率的影响,结果如图6所示。
由图6可见,当反应体系液固比较低时,增大液固比有利于人造白钨的分解浸出。当液固比超过7.5:1后,WO3浸出率达到97.5%以上,继续增大液固比对浸出率的提高影响不大。当浸出剂浓度一定时,较高的液固比虽可使人造白钨的分解进行得比较完全,但是过高的液固比会增大反应试剂的消耗,因此,在保证浸出效果较好的前提下,宜选用较低的液固比,最终选取液固比10:1作为较优条件并在其他因素实验中保持恒定。
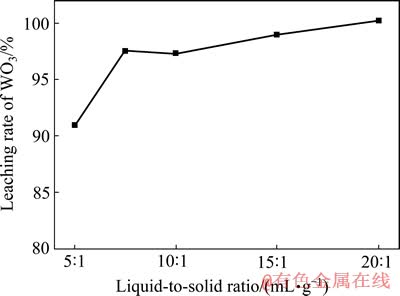
图6 液固比对人造白钨浸出率的影响
Fig. 6 Effect of liquid-to-solid ratio on leaching rate of WO3
2.5 反应时间对人造白钨浸出率的影响
在c(HCl)=1.5 mol/L、c(H2O2)=2.4 mol/L、液固比10:1和反应温度30 ℃的条件下,考察保温时间对人造白钨浸出率的影响,结果如图7所示。
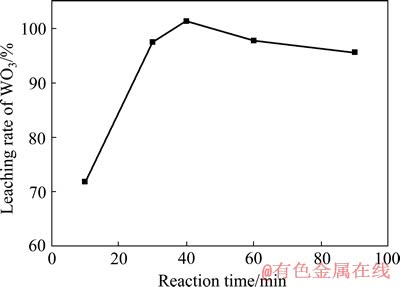
图7 反应时间对人造白钨浸出率的影响
Fig. 7 Effect of reaction time on leaching rate of WO3
由图7可以看出,在上述反应条件下,分解反应速率较快,初始阶段,随着反应时间的延长,人造白钨浸出率不断提高,当反应时间为40 min时,人造白钨分解率已经达到100%。继续延长时间,溶液中可溶性钨的含量略有下降,同样可能是双氧水分解导致浓度降低以及过氧钨酸稳定性较差引起的。故最终确定最优的反应时间为40 min。
3 结论
1) 利用双氧水易与钨酸络合生成可溶性过氧钨酸的特性,提出HCl-H2O2协同浸出体系,以破除CaWO4酸分解过程中生成的钨酸阻滞膜,促进室温及常压条件下人造白钨的分解。
2) 双氧水协同盐酸分解人造白钨的优化工艺条件如下:c(H2O2)=2.4 mol/L,c(HCl)=1.5 mol/L,反应温度30 ℃,液固比(mL/g)10:1,反应时间40 min。在此条件下,人造白钨的分解率达到100%。新方法中盐酸用量大幅降低,盐酸挥发也得到较好的避免。
REFERENCES
[1] 李俊萌. 中国钨矿资源浅析[J]. 中国钨业, 2009, 24(6): 9-13.
LI Jun-meng. On the characteristics and utilization of China’s tungsten resources[J]. China Tungsten Industry, 2009, 24(6): 9-13.
[2] MARTINS J I, LIMA  L F C, MOREIRA A, COSTAET S C. Tungsten recovery from alkaline leach solutions as synthetic scheelite[J]. Hydrometallurgy, 2007, 85(2): 110-115.
L F C, MOREIRA A, COSTAET S C. Tungsten recovery from alkaline leach solutions as synthetic scheelite[J]. Hydrometallurgy, 2007, 85(2): 110-115.
[3] MARTINS J I, MOREIRA A, COSTA S. Leaching of synthetic scheelite by hydrochloric acid without the formation of tungstic acid[J]. Hydrometallurgy, 2003, 70(1): 131-141.
[4] LUO L, MIYAZAKI T, SHIBAYAMA A, YEN W, FUJITA T. A novel process for recovery of tungsten and vanadium from a leach solution of tungsten alloy scrap[J]. Minerals Engineering, 2003, 16(7): 665-670.
[5] LUO L, KEJUN L, SHIBAYAMA A, YEN W, FUJITA T, SHINDO O, KATAI A. Recovery of tungsten and vanadium from tungsten alloy scrap[J]. Hydrometallurgy, 2004, 72(1): 1-8.
[6] 李洪桂, 羊建高, 李 昆. 钨冶金学[M]. 长沙: 中南大学出版社, 2010.
LI Hong-gui, YANG Jian-gao, LI Kun. Tungsten metallurgy[M]. Changsha: Central South University Press, 2010.
[7] 方 奇. 苛性钠压煮法分解白钨矿[J]. 中国钨业, 2001, 16(5/6): 80-81.
FANG Qi. Decomposition of scheelite with NaOH in autoclaving[J]. China Tungsten Industry, 2001, 16(5/6): 80-81.
[8] 曹才放, 赵中伟, 刘旭恒, 王小波. 硅酸钠分解白钨矿的热力学研究[J]. 中国有色金属学报, 2012, 22(9): 2636-2641.
CAO Cai-fang, ZHAO Zhong-wei, LIU Xu-heng, WANG Xiao-bo. Thermodynamic study on decomposition of scheelite with sodium silicate[J]. The Chinese Journal of Nonferrous Metals, 2012, 22(9): 2636-2641.
[9] 宁 华. 苏打压煮钨矿工艺及其发展[J]. 稀有金属与硬质合金, 1996(4): 44-48.
NING Hua. Progress of tungsten soda digesting technology[J]. Rare Metals and Cemented Carbides, 1996(4): 44-48.
[10] 彭少方. 钨冶金学[M]. 北京: 冶金工业出版社, 1981: 34-49.
PENG Shao-fang. Tungsten metallurgy[M]. Beijing: Metallurgical Industry Press, 1981: 34-49.
[11] 廖利波, 董 伟. 酸法工艺处理白钨精矿的最新进展[J]. 稀有金属与硬质合金, 2005, 33(3): 29-31.
LIAO Li-bo, DONG Wei. The latest development of the acid process for scheelite concentrate[J]. Rare Metals and Cemented Carbides, 2005, 33(3): 29-31.
[12] 彭少方, 向 兰, 张 昭. 超声振动下盐酸浸出白钨矿的研究[J]. 中国钨业, 1989(2): 7-13.
PENG Shao-fang, XIANG Lan, ZHANG Zhao. Study of scheelite leaching with HCl assisted by ultrasonic wave[J]. China Tungsten Industry, 1989(2): 7-13.
[13] 卢友中, 曾青云, 陈庆根. 微波辅助碱分解低品位黑(白)钨精矿[J]. 矿产综合利用, 2009(5): 20-24.
LU You-zhong, ZENG Qing-yun, CHEN Qing-gen. Alkaline decomposition of combined concentrate of scheelite and wolframite under microwave-assisted condition[J]. Multipurpose Utilization of Mineral Resources, 2009(5): 20-24.
[14] KAHRUMAN C, YUSUFOGLU I. Leaching kinetics of synthetic CaWO4 in HCl solutions containing H3PO4 as chelating agent[J]. Hydrometallurgy, 2006, 81(3/4): 182-189.
[15] KALPAKLI A O, ILHAN S, KAHRUMAN C, YUSUFOGLU I. Dissolution behavior of calcium tungstate in oxalic acid solutions[J]. Hydrometallurgy, 2012, 121/124: 7-15.
[16] MARTINS J I. Leaching of synthetic scheelite by nitric acid without the formation of tungstic acid[J]. Industrial & Engineering Chemistry Research, 2003, 42(21): 5031-5036.
[17] LASSNER E, SCHUBERT W D. Tungsten: Properties, chemistry, technology of the elements, alloys, and chemical compounds[M]. New York: Kluwer Academic/ Plenum Publishers, 1999.
[18] DICKMAN M H, POPE M T. Peroxo and superoxo complexes of chromium, molybdenum, and tungsten[J]. Chemical Reviews, 1994, 94(3): 569-584.
[19] 蒋安仁, 庞 震, 顾翼东. 室温和低酸度条件下钨酸的制备[J]. 高等学校化学学报, 1987, 8(5): 403-406.
JIANG An-ren, PANG Zhen, GU Yi-dong. Preparation of tungstic acid at room temperature and low acidity[J]. Chemical Journal of Chinese University, 1987, 8(5): 403-406.
[20] 张 清, 应超燕, 余可娜, 顾东虎, 肖海湖, 吴建一. 双氧水分解速率和稳定性研究[J]. 嘉兴学院学报, 2010, 22(3): 51-53.
ZHANG Qing, YING Chao-yan, YU Ke-na, GU Dong-hu, XIAO Hai-hu, WU Jian-yi. Study on decomposition rate and stability of hydrogen peroxide[J]. Journal of Jiaxing University, 2010, 22(3): 51-53.
(编辑 陈卫萍)
基金项目:国家自然科学基金重点项目(51334008)
收稿日期:2014-03-19;修订日期:2014-06-17
通信作者:赵中伟, 教授, 博士; 电话:0731-88830476;E-mail: zhaozw@csu.edu.cn