Article ID: 1003-6326(2005)06-1285-05
Effects of rare earth element Ce on solderabilities of micron-powdered Sn-Ag-Cu solder
XUE Song-bai(薛松柏)1, YU Sheng-lin(禹胜林)2,
WANG Xu-yan(王旭艳)1, LIU lin(刘 琳)1,
HU Yong-fang(胡永芳)1, YAO Li-hua(姚立华)1
(1. College of Materials Science and Technology,
Nanjing University of Aeronautics and Astronautics, Nanjing 210016, China;
2. 14th Research Institute, China Eletronics Technology Group Corporation,
Nanjing 210013, China)
Abstract: Several important properties of the micron-powdered Sn-Ag-Cu-Ce solder, including the spreadability, spreading ratio, wetting time, and melting point, were investigated for verifying the effects of rare earth element Ce on solderabilities of micron-powdered Sn-Ag-Cu solder. The solidus and the liquidus of the micron-powdered Sn-Ag-Cu-Ce solder are 193.6℃ and 218.4℃, respectively, about 28℃ and 3℃ lower than the melting point of the block Sn-Ag-Cu solder, which reminds the existence of the surface effect of the micron-powdered solder. By adding Ce into Sn-Ag-Cu alloy, its wetting time on pure copper can be obviously decreased. For the Sn-Ag-Cu-0.03%Ce, the soldering temperature is 250℃, and the wetting time on pure copper is close to 1s, with the soldering temperature approaching to 260℃, the wetting time is dropped to 0.8s, which is close to the wetting time, 0.68s, of Sn-Pb solder at 235℃.
Key words: lead-free solder; Sn-Ag-Cu-Ce alloy; wetting time; solidus; liquidus CLC
number: TG146.1 Document code: A
1 INTRODUCTION
With approaching the deadline (July 1, 2006) of WEEE and RoHS, many investigations and applications about new lead-free solders were progressed with a lot of achievements worldwide. During developing novel lead-free solders, all the assessment standards of the solders are based on the properties of traditional Sn-Pb solders. Nowadays, the solders of Sn-Ag-Cu series are generally accepted as the main alloy series for replacing the Sn-Pb solders[1, 2]. Since the melting point of Sn-Ag-Cu solders is about 38℃ higher than that of Sn-Pb solders, as well as the wettability and spreadability of Sn-Ag-Cu solders are poor than those of Sn-Pb solders, some limitations need to be overcome in the industry application of Sn-Ag-Cu solders.
With decreasing the size of metal or alloy particles from micron to nanometer, a series of special properties, especially the decrease of the melting point, will emerge[2, 3]. However, due to the difficulties in preparing Sn-Ag-Cu nano or micron powder, the reports regarding Sn-Ag-Cu micron- (or nano-) powdered solder are very limited, especially no report can be referred to the effect of Ce on the properties of Sn-Ag-Cu- Ce micron (or nano) powder.
In the present study, the fine Sn-Ag-Cu-Ce powder was manufactured by adopting a special method with the help of laser beam. The solidus, wettability and spreadability of the fine solder powder were investigated in order to assess the effect of Ce on the properties of lead-free Sn-Ag-Cu- Ce micron- (or nano-) powdered solder.
2 EXPERIMENTAL
2.1 Materials
The base metal for testing was pure copper. The lead-free solder was Sn-Ag-Cu-Ce alloy which was composed of 3.5%Ag, 1.0%Cu, 94.0%Sn, 0.05%Ce and 0.5%(mass fraction) other elements. Sn-Ag-Cu-Ce alloy was produced by means of traditional smelting method, and then made into fine spherical powders by a special laser-aided technique. At the same time, bar solders of Sn-Ag-Cu and Sn-Ag-Cu-Ce alloy were obtained as reference materials.
2.2 Spreadability test
All spreadability tests were performed according to GB/T 11364—1989 (Test method of spreadability and clearance filling ability for filler metal), referred to correlative prescribes of GB/T 3131—2001 (Tin-lead solder).
2.3 Test of wettability time
All tests of wettability time were carried out according to JIS Z 3198: 2003 (Test methods for lead-free solders,Part 4: Methods for solderbility test by a wetting balance method and a contact angle method) with SAT-5100 Solder Checker (Rhesca Corp., Japan), as shown in Fig.1. After immerging test piece into melted solder to some depth, the piece will be supported mainly by the interfacial intension between solder and the testing piece surfaces and the flotage. Assuming the flotage of dipped piece could be ignored, the variety of contact angle(θ) between the piece and the solder can be described as Fig.2(①-⑥) whether the piece is wetted or not.

Fig.1 SAT-5100 Solder Checker
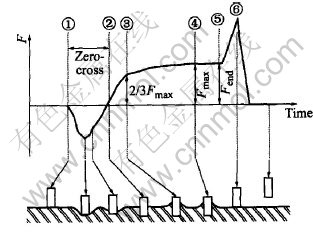
Fig.2 Test principal for determining wetting force and wetting time
3 RESULTS AND DISCUSSION
The SEM morphology of Sn-Ag-Cu-Ce powder is shown in Fig.3. It can be seen that the diameters of particles of Sn-Ag-Cu-Ce are between 1-20μm. On a smooth sphere particle, elemental compositions were analyzed by EDS, and the results are listed in Table 1.

Fig.3 SEM image of Sn-Ag-Cu-Ce powder
Table 1 Elemental composition of powder(mass fraction, %)

Usually, Sn-Ag-Cu alloy is difficult to make into spherical powders without rare earth element Ce[4-6]. Because the Ce in Sn-Ag-Cu-Ce alloy segregates at the grain boundary, as an interface activator, Sn-Ag-Cu alloy is easy to be made into spherical powders. The optimum Ce addition is about 0.03%(mass fraction).
The solidus and liquidus of powder solder of Sn-Ag-Cu-Ce are determined to be 193.6℃ and 218.4℃ respectively, by DSC (Perkin-Elmer, USA), as shown in Fig.4 and Fig.5. One of the characteristic thermodynamic properties of nano particle is the decrease of its melting point in comparison with the same block material[3]. Although, the laser-aided powder preparing method can be applied to produce a lot of metallic nano powders, in present study the particle size of Sn-Ag-Cu-Ce alloy is only micron scale with the same powder process. Even so, the solidus and liquidus temperatures of micron Sn-Ag-Cu-Ce powder are 193.6℃ and 218.4℃, respectively, 28℃ and 3℃ lower than 221℃, the melting point of the conventional block Sn-Ag-Cu lead-free solder. Compared with the solidus temperature of the block Sn-Ag-Cu-Ce solder (218 ℃)[7], that of micron-powdered Sn-Ag-Cu-Ce is 24℃ lower, which could be attributed to the reduction in particle size.
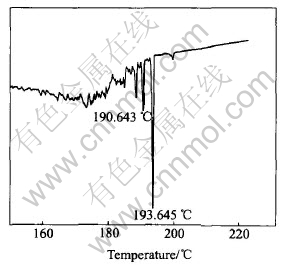
Fig.4 Solidus of Sn-Ag-Cu-Ce powder solder
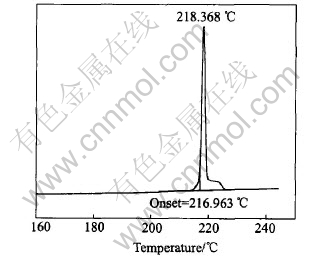
Fig.5 Liquidus of Sn-Ag-Cu-Ce powder solder
Possibly, with the reduction of particle size, atomic number on interface increases, the particle surface energy level and surface tension increases[8 -12], which results in the change of some chemo-physical properties of the particles, i.e. the surface effect. Surface effect will lead to increasing surface activity of nano particles, which changes not only the transportation and the configuration of surface atoms of the particles, but also spin construction of surface electron and surface energy spectrum. The surface effect results in the instability of active nano particles, which is the main difficulty in developing nano-powdered Sn-Ag-Cu-Ce solder.
Sn-Ag-Cu-Ce alloy powder in spherical shape is especially suitable for manufacturing soldering paste, because the solidus of Sn-Ag-Cu-Ce alloy powder is about 28℃ lower than that of block Sn-Ag-Cu-Ce alloy, and only 10℃ higher than that of Sn-Pb solder (183℃)[7, 9, 11]. If the diameter of micron Sn-Ag-Cu-Ce powder is further minimized, a novel lead-free solder with the solidus close to (even below) 183℃ could be developed in the future. Namely it is a new possible approach to developing novel applicable lead-free solders.
The solderability test results (spreading on pure copper at 235℃) are shown in Table 2. As mentioned in Table 2, both the spreading area and spreading ratio of micron-powdered Sn-Ag-Cu-Ce solder are 10% larger than that of block solder of Sn-Ag-Cu-Ce, which illustrates that the surface effect of the micron powder is remarkable and favorable for improving spreadability and solderability.
Table 2 Experimental results of spreadability and spreading ratio of micron-powdered Sn-Ag-Cu-Ce solder compared with block solder

The wetting times of Sn-Ag-Cu on pure copper at different Ce contents and temperatures were determined and shown in Figs.6-11 according to the standard of JIS Z 3198: 2003 by means of the instrument of SAT-5100 Solder Checker. From Fig.6 to Fig.11, the wetting times of Sn-Ag-Cu and Sn-Ag-Cu-Ce alloy on pure copper tend to decrease with the increase of experimental temperature. Furthermore, the wetting times decrease obviously by adding rare earth element Ce. The optimum Ce addition is about 0.03%.
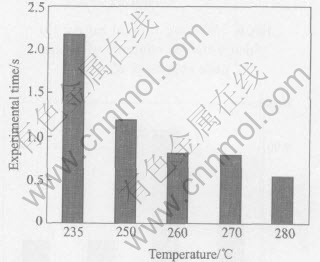
Fig.6 Variety of wetting time of Sn-Ag-Cu with temperature
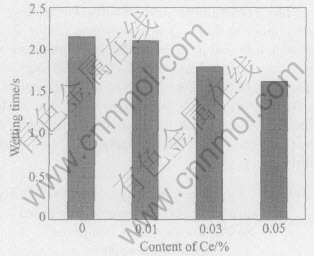
Fig.7 Wetting time of Sn-Ag-Cu added different contents of Ce on pure copper at 235℃
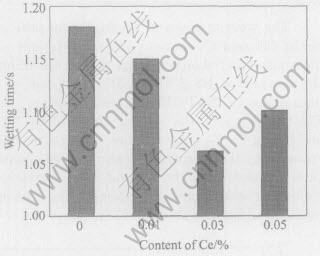
Fig.8 Wetting time of Sn-Ag-Cu added different contents of Ce on pure copper at 250℃
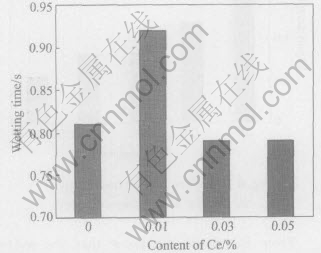
Fig.9 Wetting time of Sn-Ag-Cu added different contents of Ce on pure copper at 260℃
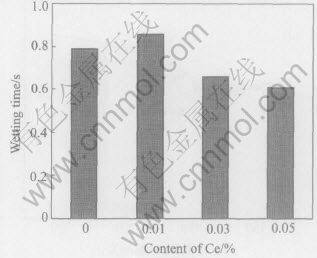
Fig.10 Wetting time of Sn-Ag-Cu added different contents of Ce on pure copper at 270℃
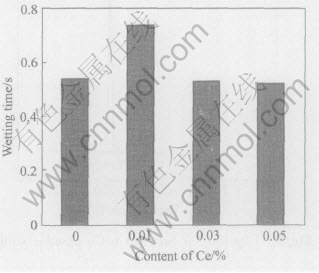
Fig.11 Wetting time of Sn-Ag-Cu added different contents of Ce on pure copper at 280℃
From Fig.6 we can know that the wetting time of Sn-Ag-Cu-Ce alloy on pure copper is less than 1s at the temperatures high than 260℃, which meets the needs of automatic soldering technology in electronic industry(i.e. t0〈1s) very well, as required by IPC/EIA J-STD-003B-2004. When the soldering temperature is below 260℃, such a technical demand cannot be satisfied. Furthemore, the wettability of Sn-Ag-Cu alloy is notably improved by adding rare earth element Ce. Especially when the content of Ce in Sn-Ag-Cu-Ce alloy is about 0.03%, the wetting time is about 1s at 250℃ and below 0.8s at 260℃(close to 0.68s, the wetting time of Sn-Pb solder at 235℃). As we know, the mechanical properties of Sn-Ag-Cu alloy are better than those of Sn-Pb solder, as well as the relatively poor wettability of Sn-Ag-Cu alloy can be improved by means of adding Ce[13 -16], which provides the experimental basis for the lead-free solders in reflow soldering application.
4 CONCLUSIONS
1) The wetting time of Sn-Ag-Cu-Ce alloy on pure copper can be reduced by adding rare earth element Ce. Especially when the content of Ce is about 0.03%, the wetting time is close to 1s at 250℃ and below 0.8s at 260℃ (0.68s for conventional Sn-Pb solder at 235℃), which is suitable for automatic soldering in electronic industry as required by IPC/EIA J-STD-003B-2004.
2) The solidus and liquidus temperatures of micron-powdered Sn-Ag-Cu-Ce solder are 193.6℃ and 218.4℃, respectively, about 28℃ and 3℃ lower than that of block Sn-Ag-Cu alloy. As well as, the solidus temperature of Sn-Ag-Cu-Ce solder is reduced by about 24℃, which can be attributed to the reduction in particle size, i.e. the surface effect.
3) The spreadability and spreading ratio of the micron-powdered Sn-Ag-Cu-Ce solder can be obviously improved in comparison with the same block solder. The spherical powder of Sn-Ag-Cu-Ce alloy, with a gradient particle size distribution, is particularly suitable to manufacture soldering paste.
REFERENCES
[1]SHI Yao-wu, XIA Zhi-dong, CHEN Zhi-gang, et al. New advances in solders research for electronic assembly [J]. Electronics Process Technology, 2001, 22(4): 139-143.(in Chinese)
[2]Hunt C. Technology Mission to Assess the Status of Lead-free Soldering in Japan [R]. NPL Report MATC(A)12, March, 2001.
[3]ZHANG Zhi-kun, CUI Zuo-lin. Nanometer Technology And Nanometer Materials [M]. Beijing: National Defence Industry Press, 2000.(in Chinese)
[4]DU Ting. Physical-chemistry effect of rare earth elements on metallic materials [J]. Acta Metallurgica Sinica, 1997, 33(1): 69-77.(in Chinese)
[5]XUE Song-bai, YU Sheng-lin. Preparation and characterization on micron powder solder of Sn-Ag-Cu-RE [J]. Transactions of the China Welding Institution, 2004, 25(6): 1-3.
[6]XUE Song-bai, CHEN Wen-hua, L Xiao-chun, et al. Mechanism of brazing flux reacting with oxide film of LY12 aluminum alloy [J]. The Chinese Journal of Nonferrous Metals, 2004, 14(4): 543-547.(in Chinese)
[7]Miller C M, Anderson I E, Smith J F. A viable tin-lead solder substitute: Sn-Ag-Cu [J]. Journal of Electronic Materials, 1994, 23(7): 595-601.
[8]YU Da-quan, ZHAO Jie, WANG Lai. Wetting properties of Sn-9Zn solder alloy with trace rare earth elements [J]. The Chinese Journal of Nonferrous Metals, 2003,13(4): 1001-1004.(in Chinese)
[9]QIAO Zhi-yu, XIE Yu-nan, CAO Zhan-ming. Design of lead-free solder alloy and alloy phase diagram calculation [J].The Chinese Journal of Nonferrous Metals, 2004,14(11): 1789-1798.(in Chinese)
[10]LIU Chun-lei, JIN Zhan-peng, LIU Hua-shan. Application of CALPHAD in soldering of electric materials [J]. The Chinese Journal of Nonferrous Metals, 2003, 13(6): 1343-1349.(in Chinese)
[11]DAI Zhi-feng, HUANG Ji-hua. Research and development of lead-free Sn-Zn based solders in microelectronics assembly [J]. Electronics Process Technology, 2004, 25(1): 5-8.(in Chinese)
[12]Kim Y S, Kim K S. Effect of composition and cooling rate on microstructure and tensile properties of Sn - Zn - Bi alloys [J]. Journal of Alloys and Compounds, 2003, 352(2): 237 - 245.
[13]XUE Song-bai, QIAN Yi-yu, DONG Jian. Equivalent activity coefficient phenomenon of cerium reacting with lead or bismuth in Ag, Cu and Zn alloy [J]. Journal of Rare Earths, 2002, 20(6): 626-629.
[14]XUE Song-bai, MA Xin, QIAN Yi-yu. Thermodynamic assessment of interaction relation between lanthanum and constituent elements in Sn-Pb alloy [J]. Journal of Rare Earths, 2001, 19(2): 107-109.
[15]Wu C M L, Yu D Q, Law C M T. Microstructure and mechanical properties of new lead-free Sn-Cu-RE solder alloys [J]. Journal of Electronic Materials, 2002, 31(9): 928-932.
[16]Wu C M L, Yu D Q, Law C M T. The properties of Sn-9Zn lead-free solder alloys doped with trace rare earth elements [J]. Journal of Electronic Materials, 2002, 31(9): 921-927.
(Edited by YUAN Sai-qian)
Received date: 2005-07-05; Accepted date: 2005-10-17
Correspondence: XUE Song-bai, Professor, PhD; Tel/Fax: +86-25-84895790; E-mail: xuesb@nuaa.edu.cn