CaCl2-NaCl熔盐中电解氧化物制备Ti-Ge金属间化合物
来源期刊:中国有色金属学报(英文版)2018年第11期
论文作者:王银帅 邹星礼 鲁雄刚 李尚书 郑凯 汪淑娟 许茜 周忠福
文章页码:2351 - 2359
关键词:Ti-Ge金属间化合物;氧化物;电解脱氧;熔盐
Key words:Ti-Ge intermetallics; oxides; electrodeoxidation; molten salt
摘 要:采用电解脱氧工艺,以不同比例的TiO2和GeO2混合物为前驱体,在电压为3.0 V、温度为800 °C的电解条件下,制备Ti-Ge(TixGey)金属间化合物。在同样电解条件下,以5TiO2-3GeO2 或 5CaTiO3-3GeO2为前驱体时,均可电解得到Ti5Ge3金属间化合物。获得的电解产物表现出相对均匀的晶粒结构。此外,初始原料(TiO2/GeO2)的摩尔比对最终电解产物有非常大的影响。根据实验数据,详细阐述电解脱氧制备TixGey金属间化合物的相关反应机理。结果表明,电解脱氧工艺是一种环境友好的制备TixGey金属间化合物的方法。
Abstract: Titanium germanium intermetallics (TixGey)were directly prepared from titanium oxide (TiO2) and germanium oxide (GeO2) powders mixture by using an electrodeoxidation process. The electrochemical experiment was carried out in a molten flux CaCl2-NaCl at 800 °C with a potential of 3.0 V. The results show that monolithic germanide Ti5Ge3 intermetallic can be directly produced from TiO2-GeO2 or CaTiO3-GeO2 precursors (both molar ratios are 5:3), and the obtained Ti5Ge3 powders exhibit homogenous particle structure. In addition, the phase composition of the final product can be dramatically affected by the initial molar ratio of TiO2 to GeO2. The reaction mechanism of the electrodeoxidation process was discussed based on the experimental results. It is suggested that the electrodeoxidation process is an environmentally friendly method for the preparation of Ti-Ge intermetallics.
Trans. Nonferrous Met. Soc. China 28(2018) 2351-2359
Yin-shuai WANG1, Xing-li ZOU1,2, Xiong-gang LU1, Shang-shu LI1, Kai ZHENG1, Shu-juan WANG1, Qian XU1, Zhong-fu ZHOU1,3
1. State Key Laboratory of Advanced Special Steel, Shanghai Key Laboratory of Advanced Ferrometallurgy, School of Materials Science and Engineering, Shanghai University, Shanghai 200072, China;
2. Center for Electrochemistry, Department of Chemistry, The University of Texas at Austin, Austin, Texas 78712, USA;
3. Institute of Mathematics and Physics, Aberystwyth University, Aberystwyth SY23 3BZ, United Kingdom
Received 16 October 2017; accepted 24 December 2017
Abstract: Titanium germanium intermetallics (TixGey)were directly prepared from titanium oxide (TiO2) and germanium oxide (GeO2) powders mixture by using an electrodeoxidation process. The electrochemical experiment was carried out in a molten flux CaCl2-NaCl at 800 °C with a potential of 3.0 V. The results show that monolithic germanide Ti5Ge3 intermetallic can be directly produced from TiO2-GeO2 or CaTiO3-GeO2 precursors (both molar ratios are 5:3), and the obtained Ti5Ge3 powders exhibit homogenous particle structure. In addition, the phase composition of the final product can be dramatically affected by the initial molar ratio of TiO2 to GeO2. The reaction mechanism of the electrodeoxidation process was discussed based on the experimental results. It is suggested that the electrodeoxidation process is an environmentally friendly method for the preparation of Ti-Ge intermetallics.
Key words: Ti-Ge intermetallics; oxides; electrodeoxidation; molten salt
1 Introduction
Titanium alloys are very attractive for the aerospace and chemical industries due to their lower density, high specific strength, excellent corrosion resistance, high melting point and good thermal property [1-4]. Moreover, the addition of IVA-group element into Ti-based alloys can further improve their corrosion resistance, mechanical property, and high temperature behavior [5-10]. The use of Ge alloys (i.e., germanides) for advanced applications in modern device technology is of current interest. Among these prospective applications, germanides are of interest in forming Schottky contacts on Ge [11]. FLEISCHER [7] has reported that Ti5Ge3 intermetallic compounds still have high strength at high temperature. ZAREMBO et al [9] and COLINET and TEDENAC [8] have studied the thermodynamic and mechanical properties of Ti-Ge system. It was suggested that Ti-Ge intermetallic compounds have excellent mechanical and thermodynamic properties. The Ti-Ge phase diagram is shown in Fig. 1 [12]. Obviously, three intermetallic compounds, i.e., Ti5Ge3, Ti6Ge5 and TiGe2, are stable in the Ti-Ge system. Based on the critical review of MURRAY [13] and the previous work [9,10], it is also concluded that the compounds Ti5Ge3, Ti6Ge5 and TiGe2 are stable. Generally, the conventional process used to produce metal germanides commonly involves arc melting of the ultrapure elements Ti and Ge, which always suffered from high energy costs [10], because the conventional processes used for the preparation of Ge involve the reduction of germanium oxides or sulfides by hydrogen or carbon [14], and pure Ti is mainly produced by the Kroll process [15].
Direct extraction of metals and/or alloys from compounds without the production of hazardous substance is the trend in the future [16]. Processes with potential for reducing or even avoiding CO2 emissions such as the electrodeoxidation technology would be especially desirable [5,17,18]. In recent years, the electrodeoxidation technologies have been used to produce a large number of metals and alloys in molten salts [19]. A variety of electrodeoxidation processes such as the Fray-Farthing-Chen (FFC) process and the solid oxide membrane (SOM)-assisted electrodeoxidation process are currently being investigated [15,19-25]. It has been proved that the electrodeoxidation of solid oxides in molten salts is an efficient and rapid approach for extracting metals, alloys and semiconductors [22]. The key innovation of this process is based on the thermodynamic fact that the decomposition voltages of many solid oxides are lower than those of the inorganic molten salts at high temperatures, ranging from 600 to 900 °C [15,19]. Therefore, based on the low decomposition voltage of TiO2 and GeO2, it is reasonable to believe that Ti-Ge intermetallics can be directly prepared from TiO2 and GeO2 powders mixture at 800 °C with a potential of 3.0 V by using the molten salt electrodeoxidation process.
The aim of this work is to report the success of applying the electrodeoxidation process to the preparation of titanium-germanium compounds directly from mixed TiO2-GeO2 powders with different molar ratios (5:3, 6:5, 1:2) in molten CaCl2-NaCl. The effects of the stoichiometry of the initial mixtures on the characteristics of the final products were investigated. In addition, direct electrochemical preparation of Ti5Ge3 from CaTiO3 and GeO2 was also investigated. Taking Ti5Ge3 as an example, the specific reaction process and the formation mechanism were also described in details.

Fig. 1 Phase diagram of Ti-Ge system [12]
2 Experimental
Commercially available powder of titanium oxide, TiO2 (Aldrich, ≥99.99%) and germanium oxide, GeO2 (Aldrich, ≥99.99%) were used as raw materials. The two reagent grade oxide powders were mixed at different stoichiometric ratios and ball-milled with anhydrous alcohol for 10 h to ensure the maximum homogenization of the constituents. And about 0.3 g of the mixture was pressed to form a cylindrical pellet (10 mm in diameter and 3 mm in thickness) under a pressure load of 15 MPa. The pellet was then sintered in air at 900 °C for 4 h in a muffle furnace with a heating rate of 5 °C/min to obtain adequate strength and the desired porosity for the electrodeoxidation process. The sintered oxide pellet was sandwiched by two porous nickel foils and then attached to a Fe-Cr-Al alloy wire as a cathode. The anode was prepared by connecting a 150 mm-long and 10 mm- diameter graphite rod to a Fe-Cr-Al alloy wire. High purity CaCl2 was produced by vacuum drying of calcium chloride dihydrate CaCl2·2H2O (99% purity) [26]. The eutectic mixture of NaCl-CaCl2 was prepared by ball milling the dry pure CaCl2 and NaCl (99% purity, n(CaCl2):n(NaCl)=1:1). About 160 g of the eutectic mixture was used as electrolyte in each experiment. Schematic illustration of the electrodeoxidation process for the electrosynthesis of Ti-Ge intermetallics from oxides precursors is illustrated in Fig. 2. The experiments were performed under an atmosphere of highly purified argon gas. Pre-electrolysis process was carried out before electrodeoxidation to remove the impurity contained in the molten salt. Then, the electrodeoxidation experiment was performed at 800 °C with a potential of 3.0 V. After the experiment, the samples were allowed to cool under a stream of argon gas in the furnace, then taken out of the furnace, washed with tap water to remove the residual solidified NaCl-CaCl2 and dried under vacuum at about 90 °C.

Fig. 2 Schematic illustration of electrodeoxidation process for electrosynthesis of Ti-Ge intermetallics from oxides (TiO2/GeO2) precursors
The phase composition of the starting materials and the products was characterized by X-ray diffraction (XRD, D8 Advance, Bruker) with the incidence beam angle of 2° in the range of 10°-80°. The surface morphology and elemental composition of the products were characterized by scanning electron microscopy (SEM, JEOL JSM-6700F, Japan) and energy dispersive X-ray spectroscopy (EDX, Oxford INCA EDS system), respectively. A BioLogic HCP-803 electrochemical workstation was used to control and record the current- time curve during the electrodeoxidation process.
3 Results and discussion
3.1 Influence of sintering on raw material and current features
The influence of sintering on the raw material composition was investigated firstly to confirm whether there were new compounds generated during the sintering process. The XRD pattern of the mixed oxide pellet (n(TiO2):n(GeO2)=5:3) after being sintered at 900 °C for 4 h is given in Fig. 3. It is apparent that no peaks occurred other than those of the starting oxides, which indicates that the sintering process cannot influence the composition of the pellet precursor. Figure 4 shows the current-time curve recorded during the electrolysis of 5TiO2-3GeO2 mixture pellet. Obviously, at the beginning of the electrodeoxidation process, the current increases with the expanding of the electrochemical three-phase interlines (3PIs) reaction area [23,27]. Then, the current decreases from approximately 1900 mA to around 400 mA after 30 min of electrolysis. After that, the reduction current gradually decreases and tends to be constant at a stable level (background current). It can be seen that the background current is approximately 200 mA. After being electrolyzed for different time, the XRD patterns of the pellet’s outer and inner parts are shown in Fig. 5. It can be seen from Fig. 5(a) that the outer part of the pellet reduced for 5 h mainly contains Ti5Ge3. In addition to Ti5Ge3, the inner part of the reduced pellet still contains CaTiO3, Ge and Ti6Ge5. To completely electrolyze the inner part of the pellet to form Ti5Ge3, longer electrolysis time (8 h) is needed (Fig. 5(b)). It is obvious that the electrodeoxidation process for the pellet’s interior is slower than that for the pellet’s surface. Generally, the electrodeoxidation reaction occurs in the region of electronic conductor/oxide-compounds/electrolyte 3PIs and the 3PIs expand gradually from the surface to the center of the pellet [27].

Fig. 3 XRD pattern of 5TiO2-3GeO2 mixture pellet after being sintered at 900 °C for 4 h

Fig. 4 Typical current-time curve recorded during electrolysis of 5TiO2-3GeO2 mixture pellet under 3.0 V at 800 °C (The inset is initial part of current-time curve)
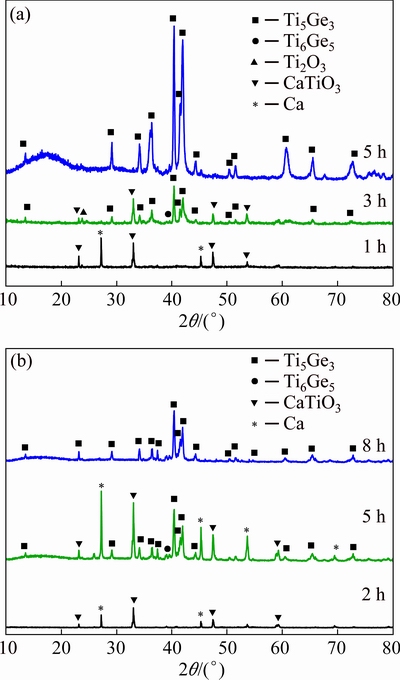
Fig. 5 XRD patterns of outer (a) and inner part (b) of 5TiO2-3GeO2 mixture pellet after being electrolyzed under 3.0 V at 800 °C for different time
3.2 Influence of reaction time and raw materials on final products
To investigate the influence of reaction time on the final products, the electrodeoxidation of mixed powders (n(TiO2):n(GeO2)=5:3) was carried out at 800 °C with a constant voltage of 3.0 V for 2, 4, 6, and 8 h, respectively. Figure 6 shows the XRD patterns of the cathode pellets after being electrolyzed for different time. Obviously, the product electrolyzed for 2 h contains Ge, but no metallic Ti phase is observed. This observation may imply that GeO2 was firstly electrodeoxidized to Ge following reaction (1):
GeO2+4e→Ge+2O2-
(ΔG800 °C=373.5 kJ/mol, E800 °C=0.97 V) (1)

Fig. 6 XRD patterns of 5TiO2-3GeO2 mixture pellet after being electrolyzed under 3.0 V at 800 °C for different time
However, during the electrodeoxidation process, TiO2 would convert into CaTiO3 following Reaction (2):
TiO2+Ca2++O2-→CaTiO3
(ΔG800 °C=-86.9 kJ/mol) (2)
The products obtained from 2-6 h electrolysis consist of Ti5Ge3, Ti6Ge5, CaTiO3 and Ti2O3 (see Fig. 6), which may suggest that Reactions (3) and (4) occurred, i.e., Ca-containing intermediate compounds (CaTiO3), were reduced to Ti/Ti2O3 [20] and CaO.
CaTiO3+4e→Ti+2O2-+CaO
(ΔG800 °C=836.7 kJ/mol, E800 °C =2.17 V) (3)
2CaTiO3+2e→Ti2O3+O2-+2CaO
(ΔG800 °C=902.0 kJ/mol, E800 °C=2.34 V) (4)
Then, CaO would dissolve into molten CaCl2-NaCl following Reaction (5), and Ti2O3 was further reduced to Ti following Reaction (6):
CaO→Ca2++O2- (5)
Ti2O3+6e→2Ti+3O2-
(ΔG800 °C=1222.5 kJ/mol, E800 °C=2.11 V) (6)
Generally, two simultaneous processes are involved during the synthesis of Ti5Ge3, i.e., (1) Ge reacts with Ti to form Ti5Ge3 and Ti6Ge5 at the experiment temperature (Reactions (7) and (8)); (2) the formed Ti6Ge5 further reacts with Ti to form Ti5Ge3 (Reaction (9)).
5Ti+3Ge→Ti5Ge3
(ΔG800 °C=-652.6 kJ/mol) (7)
6Ti+5Ge→Ti6Ge5
(ΔG800 °C=-828.1 kJ/mol) (8)
3Ti6Ge5+7Ti→5Ti5Ge3
(ΔG800 °C=-778.7 kJ/mol) (9)
Figure 7 shows the typical SEM image and its elemental maps (Ti and Ge) as well as EDS spectrum of the Ti5Ge3 product synthesized from a mixed powder pellet (n(TiO2):n(GeO2)=5:3). It can be seen that Ti and Ge elements are uniformly distributed in the product. In addition, Fig. 6 also demonstrates that CaTiO3 occurred as an intermediate product during the synthesis of Ti5Ge3. Therefore, based on this observation, we try to use CaTiO3-GeO2 as precursors to produce Ti5Ge3. Figure 8 shows the current-time curve of the electrolysis of 5CaTiO3-3GeO2 mixture pellet. It is found that the electrolysis of 5CaTiO3-3GeO2 only needs 6 h. The current efficiency which can only be used as a general evaluation for the electrolysis process is defined as the ratio of the theoretical charge for reducing all oxides contained in the pellet and the total charge passed through the electrolytic cell. The current efficiency for Ti5Ge3 production from 5TiO2-3GeO2 electrolyzed for 8 h is calculated to be 37.8%. As a comparison, the current efficiency for Ti5Ge3 production from 5CaTiO3-3GeO2 electrolyzed for 6 h is calculated to be 40.2%. Figure 9(a) shows the XRD patterns of the mixed CaTiO3 and GeO2 powders (n(CaTiO3):n(GeO2)=5:3) after being electrolyzed for different time. Apparently, the reaction process of CaTiO3-GeO2 is similar to that of TiO2-GeO2, and Ti5Ge3 can be obtained as final products. Figure 9(b) shows the XRD patterns of Ti5Ge3 obtained from different raw materials, i.e., CaTiO3- GeO2 and TiO2-GeO2. Obviously, the two Ti5Ge3 are similar. Figures 9(c) and (d) show the typical SEM image and EDS spectrum of the Ti5Ge3 synthesized from 5CaTiO3-3GeO2 powder. The SEM image indicates that the Ti5Ge3 particles possess homogenous structure, and there is no other impurity elements contained in the product (Fig. 9(d)).

Fig. 7 SEM image of completely electrolyzed product (electrolyzed for 8 h) obtained from mixed powder pellet (n(TiO2):n(GeO2)= 5:3) under 3.0 V at 800 °C (a), elemental maps of Ti (b) and Ge (c) corresponding to (a), and EDS spectrum (d) measured over image (a)

Fig. 8 Current-time curve recorded during electrolysis of 5CaTiO3-3GeO2 mixture pellet under 3.0 V at 800 °C (The inset is initial part of current-time curve)

Fig. 9 XRD patterns of mixed 5CaTiO3-3GeO2 powder pellet electrolyzed for different time under 3.0 V at 800 °C (a), XRD patterns of final products obtained from different raw materials under 3.0 V at 800 °C (b), SEM image of completely electrolyzed products (electrolyzed for 6 h) from mixed 5CaTiO3-3GeO2 powder pellet under 3.0 V at 800 °C (c) and EDS spectrum (d) of obtained Ti5Ge3 product corresponding to (c)
3.3 Electrochemical synthesis of TixGey
Electrolysis of TiO2-GeO2 pellets with different starting stoichiometries, i.e., molar ratios of TiO2 to GeO2=5:3, 6:5, 1:2, was also carried out at 800 °C for 8 h with a potential of 3.0 V. The XRD patterns and the SEM images of the completely electrolyzed products are shown in Figs. 10 and 11, respectively. A summary of germanide phases formed after electrolysis and the serial number of SEM images/XRD patterns with different starting molar ratios of TiO2 to GeO2 are listed in Table 1. Figure 10 reveals that a single-phase Ti5Ge3 intermetallic is obtained when the TiO2/GeO2 molar ratio is 5:3, and two germanide phases, i.e., Ti6Ge5 and Ti5Ge3, are formed as the product when TiO2/GeO2 molar ratio equals 6:5. The final product consists of Ti6Ge5 and pure metal Ge when TiO2/GeO2 molar ratio is 1:2. It should be noted that the XRD results cannot prove that the oxygen content has been completely removed during electrolysis, a small amount of oxygen component may still exist in the products due to the fact that the molten salt electrolysis is difficult to completely remove the oxygen element. Figure 11 reveals that all final products possess porous morphologies. Additionally, the microstructures of the products obtained from different starting stoichiometries have slight differences. As shown in Fig. 11, the single-phase Ti5Ge3 obtained from 5TiO2-3GeO2 exhibits homogenous particle structure, which shows a slight difference from the SEM images (Figs. 11(b) and (c)) of the other two products. The reason may be attributed to their different phase compositions.

Fig. 10 XRD patterns of products completely electrolyzed for 8 h under 3.0 V at 800 °C from powder mixtures with different starting molar ratios of TiO2 to GeO2
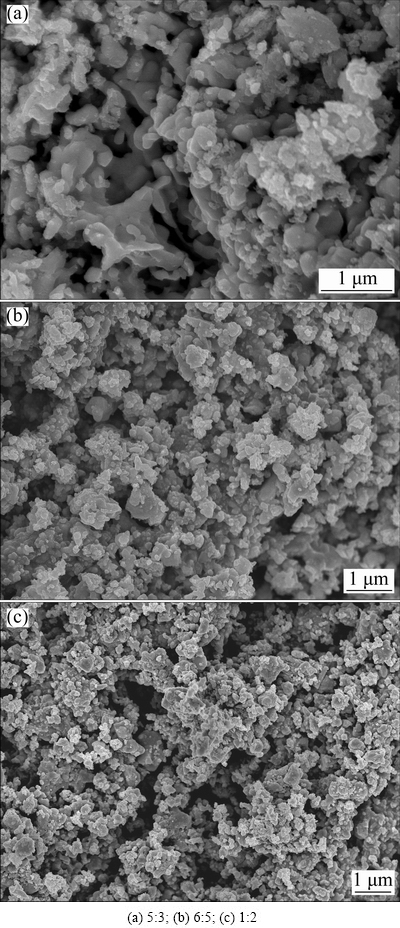
Fig. 11 SEM images of products completely electrolyzed for 8 h under 3.0 V at 800 °C with different starting molar ratios of TiO2 to GeO2
Table 1 Summary of germanide phases of electrolytic products and serial number of SEM images/XRD patterns with different starting molar ratios of TiO2 to GeO2 under 3.0 V at 800 °C

3.4 Formation mechanisms
Based on the experimental result, a mechanism for the formation of Ti5Ge3 from TiO2/GeO2 by the electrodeoxidation process was suggested and summarized in Fig. 12. During the electrolysis, the intermetallic Ti5Ge3 was formed following a multi-step process, i.e., at the beginning of the electrolysis, when the applied voltage is higher than the decomposition potential of GeO2 (GeO2 0.97 V, TiO2 1.94 V at 800 °C [28]), GeO2 was firstly electrodeoxidized to Ge at 800 °C. Simultaneously, CaTiO3 was formed during the electrodeoxidation process. Subsequently, CaTiO3 compounds were reduced to Ti-Ti2O3 and CaO. Then, Ti2O3 was reduced to Ti, the formed Ti and Ge would react to form Ti6Ge5 and Ti5Ge3. It is worth noting that there is a general trend that Ti6Ge5 was formed firstly during the electrolysis process, then the formed Ti6Ge5 would further react with Ti to form Ti5Ge3 if there is enough Ti. This experimental observation is supported by previous thermodynamic considerations [9,11,29]. In addition, the Gibbs free energy change of the reaction 5Ti+3Ge→Ti5Ge3 is negative at the electrolysis temperature [11], which means that the electrodeoxidation-generated Ti and Ge can react to form Ti5Ge3. Therefore, it is reasonable to believe that two reaction routes, i.e., Ti, Ge→Ti5Ge3 and Ti, Ge→Ti6Ge5, Ti→Ti5Ge3 coexist during the electro- synthesis process.
It should be noted that TiGe2 was not produced during the electrolysis of 1TiO2-2GeO2, (as shown in Figs. 10(c) and 13). The foregoing study [29] suggested that if sufficient time was given for the reaction to continue, Ti6Ge5 would transform to TiGe2 by the reaction with the excess Ge available according to the reaction of 7Ge+Ti6Ge5→6TiGe2. However, the reaction is a very slow solid-state reaction at the electrolysis temperature. In the work of JAIN and KAO [10], it was suggested that hundreds of hours were needed to synthesize TiGe2 at 900 °C. Therefore, it is difficult to form TiGe2 in molten salt electrodeoxidation process because the electrolysis time is insufficient for the solid transformation from Ti6Ge5 to TiGe2.
4 Conclusions
1) TixGey intermetallic compounds were synthesized by direct electrodeoxidation of mixed TiO2-GeO2 powders in molten CaCl2-NaCl at 800 °C with a potential of 3.0 V.
2) The reaction mechanism for the synthesis of Ti5Ge3 is a multi-step process, which generally includes the electrodeoxidation of GeO2, the formation of CaTiO3 compounds, the electrodeoxidation of CaTiO3 compounds and the formation of Ti5Ge3 intermetallic.
3) Single-phase Ti5Ge3 can be obtained from 5TiO2-3GeO2 and 5CaTiO3-3GeO2.
4) The results imply that the molten salt electrodeoxidation process is a fast and environmentally friendly process for the production of TixGey intermetallic.

Fig. 12 Schematic illustration of formation mechanism of Ti5Ge3 from 5TiO2-3GeO2 during electrodeoxidation process under 3.0 V at 800 °C

Fig. 13 XRD patterns of 1TiO2-2GeO2 electrolyzed under 3.0 V at 800 °C for different time
Acknowledgments
The authors thank the Instrumental Analysis and Research Center of Shanghai University for materials characterization.
References
[1] MUTLU I. Synthesis and characterization of Ti-Co alloy foam for biomedical applications [J]. Transactions of Nonferrous Metals Society of China, 2016, 26: 126-137.
[2] YUAN Bao-guo, ZHENG Yu-bin, WANG Yu-jie, GONG Long-qing. Hydrogen absorption characteristics and microstructural evolution of TC21 titanium alloy [J]. Transactions of Nonferrous Metals Society of China, 2016, 26: 599-606.
[3] LAN Chun-bo, LI Guo, WU Yu, GUO Li-li, CHEN Feng. Effects of cold deformation on microstructure and mechanical properties of Ti-35Nb-2Zr-0.3O alloy for biomedical applications [J]. Transactions of Nonferrous Metals Society of China, 2017, 27: 1537-1542.
[4] SONG Xiao-guo, ZHANG Te, FENG Yang-ju, TAN Cai-wang, CAO Jian, ZHANG Wen-cong. Brazing of TiBw/TC4 composite and Ti60 alloy using TiZrNiCu amorphous filler alloy [J]. Transactions of Nonferrous Metals Society of China, 2017, 27: 2193-2201.
[5] MISHRA B, OLSON D L. Molten salt applications in materials processing [J]. Journal of Physics and Chemistry of Solids, 2005, 66: 396-401.
[6] WANG Hui-yuan, SI Wen-ping, LI Shi-long, ZHANG Nan, JIANG Qi-chuan. First-principles study of the structural and elastic properties of Ti5Si3 with substitutions Zr, V, Nb, and Cr [J]. Journal of Materials Research, 2010, 25: 2317-2324.
[7] FLEISCHER R L. High-strength, high-temperature intermetallic compounds [J]. Journal of Materials Science, 1987, 22: 2281-2288.
[8] COLINET C, TEDENAC J C. An ab initio study of the structural, electronic, and thermodynamic properties of Ti6Si2B and Ti6Ge2B with Fe2P-type structure [J]. Solid State Communications, 2011, 151: 1018-1021.
[9] ZAREMBO A S, KEMATICK A R J, MYERSA C E, COTTSB E J. Vaporization thermodynamics and heat capacities of Ti5Ge3 and Ti6Ge5 [J]. Journal of Alloys and Compounds, 2000, 306: 78-86.
[10] JAIN T A, KAO C R. Binary compounds in the Ge-Ti system [J]. Journal of Alloys and Compounds, 1999, 282: L9-L12.
[11] PELLEG J, ELIAHU R, BARKAI A, LEVI G. A note on the reactions in the Ti-Ge system [J]. AIP Advances, 2012, 2: 032185-01-13.
[12] LIU Dan-dan, YAN Huan-li, YUAN Xiao-ming, CHUNG Yong-sun, DU Yong, XU Hong-hui, LIU Li-bin, NASH P. Thermodynamic modeling of the Ge-Ti system supported by key experiment [J]. Thermochimica Acta, 2011, 521: 148-154.
[13] MURRAY J. Phase diagrams of binary titanium alloys [M]. Materials Park, Ohio, US: ASM International, 1987.
[14] RONG Liang-bin, HE Rui, WANG Zhi-yong, PENG Jun-jun, JIN Xian-bo, CHEN G Z. Investigation of electrochemical reduction of GeO2 to Ge in molten CaCl2-NaCl [J]. Electrochimica Acta, 2014, 147: 352-359.
[15] CHEN G Z, FRAY D J, FARTHING T W. Direct electrochemical reduction of titanium dioxide to titanium in molten calcium chloride [J]. Nature, 2000, 407: 361-364.
[16] LU Ke. The future of metals [J]. Science, 2010, 328: 319-320.
[17] ALLANORE A, FENG J, LAVELAINE H, OGLE K. The adsorption of hematite particles on steel in strongly alkaline electrolyte [J]. Journal of The Electrochemical Society, 2010, 157: E24-E30.
[18] ALLANORE A, LAVELAINE H, VALENTIN G, BIRAT J P, DELCROIX P, LAPICQUE F. Observation and modeling of the reduction of hematite particles to metal in alkaline solution by electrolysis [J]. Electrochimica Acta, 2010, 55: 4007-4013.
[19] WANG Di-hua, JIN Xian-bo, CHEN G Z. Solid state reactions: An electrochemical approach in molten salts [J]. Annual Reports on the Progress of Chemistry, 2008, 104: 189-234.
[20] ZOU Xing-li, LU Xiong-gang, ZHOU Zhong-fu, XIAO Wei, ZHONG Qin-dong, LI Chong-he, DING Wei-zhong. Electrochemical extraction of Ti5Si3 silicide from multicomponent Ti/Si-containing metal oxide compounds in molten salt [J]. Journal of Materials Chemistry A, 2014, 2: 7421-7430.
[21] ZOU Xing-li, CHEN Chao-yi, LU Xiong-gang, LI Shang-shu, XU Qian, ZHOU Zhong-fu, DING Wei-zhong. Solid oxide membrane (SOM) process for facile electrosynthesis of metal carbides and composites [J]. Metallurgical and Materials Transactions B, 2016, 48: 664-677.
[22] YIN Hua-yi, XIAO Wei, MAO Xu-hui, WEI Wei-feng, ZHU Hua, WANG Di-hua. Template-free electrosynthesis of crystalline germanium nanowires from solid germanium oxide in molten CaCl2-NaCl [J]. Electrochimica Acta, 2013, 102: 369-374.
[23] XIAO Wei, JIN Xian-bo, DENG Yuan, WANG Di-hua, HU Xiao-hong, CHEN G Z. Electrochemically driven three-phase interlines into insulator compounds: Electroreduction of solid SiO2 in molten CaCl2 [J]. Chem Phys Chem, 2006, 7: 1750-1758.
[24] ZOU Xing-li, LI Xin, SHEN Bin, LU Xiong-gang, XU Qian, ZHOU Zhong-fu, DING Wei-zhong. CeO2-Y2O3-ZrO2 membrane with enhanced molten salt corrosion resistance for solid oxide membrane (SOM) electrolysis process [J]. Metallurgical and Materials Transactions B, 2016, 48: 678-691.
[25] LI Hong-mei, SONG Qiu-shi, XU Qian, CHEN Ying, MENG Jing-chun. Electrochemical synthesis of NbC-Sn composite powder in molten chloride [J]. Transactions of Nonferrous Metals Society of China, 2017, 27: 2310-2316.
[26] ABDELKADER A M, FRAY D J. Direct electrochemical preparation of Nb-10Hf-1Ti alloy [J]. Electrochimica Acta, 2010, 55: 2924-2931.
[27] DENG Yuan, WANG Di-hua, XIAO Wei, JIN Xian-bo, HU Xiao-hong, CHEN G Z. Electrochemistry at conductor/insulator/ electrolyte three-phase interlines: A thin layer model [J]. Journal of Physical Chemistry B, 2005, 109: 14043-14051.
[28] BARIN I. Thermochemical data of pure substances [M]. Weinheim, Germany: Wile-VCH, Verlag Gm6H, 1995.
[29] PELLEG J, SHNECK R, ELIAHU R. Reactions between a Ge substrate and a sputter deposited Ti film [J]. AIP Advances, 2014, 4: 067116-01-13.
王银帅1,邹星礼1,2,鲁雄刚1,李尚书1,郑 凯1,汪淑娟1,许 茜1,周忠福1,3
1. 上海大学 省部共建高品质特殊钢冶金与制备国家重点实验室,上海市钢铁冶金新技术重点实验室,材料科学与工程学院,上海 200072;
2. Center for Electrochemistry, Department of Chemistry, The University of Texas at Austin, Austin, Texas 78712, USA;
3. Institute of Mathematics and Physics, Aberystwyth University, Aberystwyth SY23 3BZ, United Kingdom
摘 要:采用电解脱氧工艺,以不同比例的TiO2和GeO2混合物为前驱体,在电压为3.0 V、温度为800 °C的电解条件下,制备Ti-Ge(TixGey)金属间化合物。在同样电解条件下,以5TiO2-3GeO2 或 5CaTiO3-3GeO2为前驱体时,均可电解得到Ti5Ge3金属间化合物。获得的电解产物表现出相对均匀的晶粒结构。此外,初始原料(TiO2/GeO2)的摩尔比对最终电解产物有非常大的影响。根据实验数据,详细阐述电解脱氧制备TixGey金属间化合物的相关反应机理。结果表明,电解脱氧工艺是一种环境友好的制备TixGey金属间化合物的方法。
关键词:Ti-Ge金属间化合物;氧化物;电解脱氧;熔盐
(Edited by Xiang-qun LI)
Foundation item: Project (51574164) supported by the National Natural Science Foundation of China; Project (2014CB643403) supported by the National Basic Research Program of China
Corresponding author: Xing-li ZOU, E-mail: xlzou@shu.edu.cn; Xiong-gang LU, Tel: +86-21-56334042, Fax: +86-21-56335768, E-mail: luxg@shu.edu.cn
DOI: 10.1016/S1003-6326(18)64880-0

