
Mechanism of effect of rare earth oxides on high temperature corrosion resistance of weld metal
LI Zhi-yong(李志勇)1, WANG Bao(王 宝)1 , ZHANG Han-qian(张汉谦)2
1. College of Materials Science and Technology, North University of China, Taiyuan 030051, China;
2. Institute of Welding Material, Taiyuan University of Technology, Taiyuan 030024, China
Received 20 April 2006; accepted 30 June 2006
Abstract: Through the addition of rare earth oxides into the coating of electrodes, the high temperature oxidation and sulphidation resistance of weld metal were studied. The transfer mechanism of rare earth oxides from coating to the welding pool and the physicochemical reaction during the process were analyzed. With the application of scanning electron microscope and energy spectrometer, the mechanism of weld metal corrosion in air and sulphur environment were studied. The result shows that the addition of rare earth oxides can improve the high temperature resistance of weld metal, especially in sulphidation environment. The electrodes in which La2O3 is added have better performance than the electrodes in which CeO2 is added. The existence form and distribution characteristic of rare earth are defined. It is believed that the marked effect of rare earth oxides in sulphur environment is related to the corrosion mechanism.
Key words: high temperature; oxidation; sulphidation; rare earth oxide; electrode coating; weld metal
1 Introduction
China is rich in natural resource of rare earth. Many researches have been put into effect for the application of rare earth in steel with promising results. It is an important study field that the rare earth is used to improve the high temperature corrosion resistance of metal[1-3]. Study on application of rare earth on weld material is also increased. Through the addition of rare earth in electrode coating, researchers could get better structure property of weld metal[4-6]. However, the rare earth metal is liable to react with oxides, which makes it difficult to transfer in the welding arc. MICHELS reported that the addition of rare earth oxides could also improve the property of steel[7], but few studies had been put on the transfer mechanism of rare earth oxides in the high temperature welding arc, not to say the mechanism of improving the high temperature corrosion resistance of weld metal. Therefore, in this study the experiment research and theoretical analysis on this field are done. The achievement is critical both in basic theory and application.
2 Experimental
2.1 Selection of rare earth oxides for addition in electrode coating
Rare earth metal and oxides are widely used in many fields now. The main applications in steel include[1]: metamorphism to refine the metallurgical structure, grain boundary adsorption to deduce sosoloid impurity content, invigoration effect, improving high temperature mechanical property, improving high temperature corrosion resistance, etc.
It is known that La2O3, ThO2, CeO2, Y2O3 and Li2O have effect on the high temperature performance[6, 7], among which La2O3, CeO2 and Y2O3 have better performance in high temperature recycle. Because the performances of La2O3, CeO2 and Y2O3 are similar and La2O3 and CeO2 have the advantage of low cost, La2O3 and CeO2 are selected for electrode coating addition in this study.
2.2 Test materials
Eight types of electrodes were used in this test, among which A402 is applied as an comparing electrode(it is tested to be the best electrode for high temperature corrosion resistance among traditional electrodes A302 and A202[5]) and the other seven applied electrodes are Re0, Re1, Re2, Re3, Re4, Re5 and Re6, in which different amounts of rare earth oxides are added. Table 1 shows the basic chemical composition of weld metals and Table 2 shows the type and amount of rare earth oxides addition. Among them Re0 has the same basic alloy system as others with no rare earth oxides addition for the reason of comparison.
The core welding-wire applies H0Cr21Ni10. The coating applies titania type coating and the weld metal is transferred both from the wire and coating. This method solves the problem of overheat and dehiscence of coating and has the characteristic of highly activity, energy- saving and material-saving[8].
2.3 Test methods
This test applies mass loss (increase) method of high temperature oxidation and sulphidation.
2.3.1 Sample preparation
After forming the deposited metals of the four electrodes with surface welding, the samples of weld metals are prepared through cutting the deposited metals by linear cutting machine. The parameters of the process are given as follows: the diameter of electrodes of 3.2 mm; welding current of 120 A; weld beam with length of 200 mm, height of 8-10 mm and width of above 20 mm; samples with height of 4 mm, width of 10 mm and length of 20 mm.
Surfaces of the samples are treated to be without burr.
2.3.2 High temperature oxidation and sulphidation
First, the size and mass of original samples are precisely measured out. Second, the precise size and mass of the samples are measured out after every high temperature (1 200℃) cycle of oxidation and suliphi- dation. Third, the ratio of mass loss to surface area of the samples is figured out. That is the mass loss rate.
3 Results and discussion
Because the oxidation cycle will cause some stress at the surface of samples due to volume change, the oxidation film will detach when it reaches certain thickness. If there are not uniform component and severe corrosion, the growth and detach of films will reach balance. So the high temperature oxidation mass loss rate can reflect the high temperature oxidation resistance of the weld metal.
Fig.1 shows the oxidation mass loss rate versus time of the eight electrodes.
In the curves of oxidation test, the high temperature oxidation resistance of the weld metal improves with the increase of addition La2O3. However, for addition CeO2, the performance of electrode in which 1.5%CeO2 is added falls down slightly. This is different from the results of other studies. All in all, the effect of addition of rare earth oxides on high temperature oxidation resistance is not as notable as that reported by MICHELS [7]. In general, the addition of rare earth oxides in electrodes can improve the high temperature oxidation resistance of weld metal in air, but not as obvious as that reported by the reference.

Fig.1 Mass loss rate of special electrode oxidation versus time
Table 1 Chemical composition of weld metal (mass fraction, %)

Table 2 Addition amount of rare earth oxide (mass fraction, %)

Fig.2 shows the mass loss rate of the eight electrodes sulphidation versus time. For sulphidation test, among the electrode in which La2O3 is added, Re2 and Re3 have better performance than Re1; while Re2 and Re3 do not have obvious differences. This is similar to CeO2. So, with the increase of addition of rare earth oxides, the sulphidation resistance of weld metal will improve. When the addition amount reaches a certain value, the sulphidation resistance will not improve greatly. Furthermore, it is noticed that the electrodes in which La2O3 is added have better performance than the electrode in which CeO2 is added.
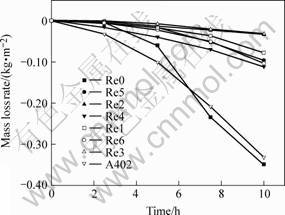
Fig.2 Mass loss rate of electrodes sulphidation versus time
4 Mechanism of effect of rare earth oxides
4.1 Transfer mechanism of rare earth oxides from coating to weld metal
Rare earth oxides will have the reaction shown in Eqns.(1) and (2) at high temperature[9]:
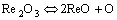 (1)
(1)
 (2)
(2)
Rare earth oxides will decompose at high temperature. Eqn.(1) will occur when TCe>3 619.0 K for CeO2 and Eqn.(2) will occur when TLa>3 718.1 K for La2O3[10]. The temperature can reach 5 000-30 000 K in the welding arc which is enough for the reaction above to occur and rare earth atom can exist in the welding arc. The active rare earth atom created in the welding arc can be easily absorbed by the liquid metal droplet surface and can be transferred to the welding pool. The reaction above can not occur in the welding pool for the temperature is about 2 140 K.
The rare earth oxides in the welding pool can react with C, S and Al besides the reaction above[6]. Some of the remain rare earth oxides transfer to the slag and some of them exist in the weld metal as the form of rare earth oxide particle.
The rare earth element and oxides transferred by different forms in the reactions above can improve the high temperature corrosion resistance, low temperature dynamic ductile, reduce the diffusion hydrogen in weld metal and so on.
4.2 Mechanism of rare earth oxides on high temperature corrosion resistance of weld metal
Using rare earth elements to improve high temperature oxidation resistance and high temperature corrosion resistance is an important research field. The researchers provide the following explains to the mechanism of it[2]: rare earth elements can increase the cohesion of film and basal body; rare earth elements can cancel the vacant lattice site forming in high temperature oxidation; rare earth can increase the diffusing speed of Cr; rare earth elements aggregate at the boundary of alloy and oxidation film which can release the stress; rare earth elements can form sosoloid in the film and improve its ability of plastic deformation.
In order to further study the effect of rare earth oxides on high temperature corrosion resistance of weld metal, scanning electron microscope (SEM) images are taken for the weld metal test samples after high temperature corrosion recycle, as shown in Fig.3.
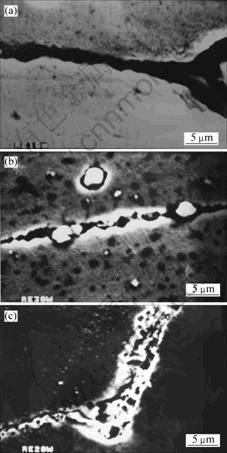
Fig.3 SEM images of grain boundary: (a) Re0; (b) Re3; (c) Re6
From Fig.3, SH1(Re0) is shown to have serious oxidation and corrosion at the grain boundary, SH2(Re6) is shown to have better oxidation and corrosion resistance at the grain boundary. MICHELS’ study shows that the addition of CeO2 can change the film structure of Cr2O3 and decrease the diffusion speed of vacancy, thus improve the protection effect[7]. In SH3(Re3), not only the decrease of high temperature oxidation is observed, but also particles are observed to exist at the grain boundary. The energy spectrum analysis proves that La and Cr are enrich in the particle. The study about La shows that La is liable to center on the grain boundary and prevent Cr3+ to diffusion outward, thus slow down the oxidation rate. La can also exist in the same form to attach the film to the base metal and decrease the detaching rate of film during high temperature oxidation recycle[11]. However, it shows no significant effect in the improving of high temperature oxidation resistance. The reason for that lies on that the alloy system can meet the requirement of entire Cr2O3 film growth rate, which is critical for the high temperature property. Thus the effect of rare oxides can not be shown.
In the sulphidation environment, the corrosion mode is selective corrosion and the grain boundary will prefer to be eroded[2]. The mechanism of corrosion is as follows:
1) In pre-corrosion stage,oxides will be formed according to M—O—S thermodynamics balance graph and then sulphides will be formed in the metal/oxides boundary or in the base metal. Sulphur is diffused through the oxides or transfer by SO2 and inner- sulphidation will occur.
2) Through inner-sulphidation, sulphur permeates to the front edge of corrosion layer and forms sulphides, which provides corrosion channel for further corrosion. When oxygen pressure grows, new oxides will form and sulphur will be substituted. The sulphur can create new corrosion channel. By the alternating corrosion mode, the corrosion speed is accelerated.
From the analysis above, it is shown that preventing the high alternating temperature corrosion from occurring at the grain boundary is critical for improving the high temperature corrosion resistance.
It is noticed in oxidation analysis and rare earth transfer mode analysis in 3.1 that rare earth element and its oxides are liable to center and distribute at the grain boundary. The reaction shown in Eqns.(3) and (4) will occur in the front edge of corrosion grain boundary in high temperature sulphidation environment [9]:
Re2O3+S+C == Re2O2S+CO (3)
2Re+3S == Re2S3 (4)
The reactions prove that the rare earth element and oxides have desulphurization effect. The reaction can decrease the alternating corrosion mode and the rare earth compound created in the grain boundary can prevent the forming of corrosion channel. The effect combines with the film attaching effect and improving Cr transfer speed can greatly improve the high temperature corrosion resistance in sulphidation environment.
5 Conclusions
1) Rare earth oxidation can obviously improve the high temperature performance in sulphidation environment. The electrodes in which La2O3 is added have better performance than the electrodes in which CeO2 is added.
2) Rare earth oxides will decompose at high temperature of welding arc and be transferred to the welding pool. The rare earth element and oxides transferred to the weld metal exist in different forms.
3) Rare earth element and its oxides are liable to center and distribute at the grain boundary and decrease the alternating corrosion mode, which can greatly improve the high temperature corrosion resistance in sulphidation environment.
References
[1] TANG Bo-gang, WANG Shi-yao. The application of rare earth on steel welding[A]. The First Conference on Rare Earth Application on Steel[C]. Baotou: China Rare Earth Society, 1982. 34-36. (in Chinese)
[2] QI Hui-bin, ZHU Ri-zhang. High Temperature Corrosion and High Temperature Corrosion Resistance Material [M]. Beijing: Aeronautics & Astronautics Press, 1985. (in Chinese)
[3] LIN Q, YE W, DU Y S. Effect of rare earth metal on properties of steels and optimization control[J]. Journal of University of Science and Technology Beijing, 1992, 14(2): 225-228. (in Chinese)
[4] LI Z Y, WANG B, ZHANG H Q. Effect of rare earth oxides on the high temperature oxidation and corrosion resistance of weld metal[J]. Chinese Rare Earth, 1999, 20(2): 57-59. (in Chinese)
[5] LI Zhi-yong, LI Jun-yue, WANG Bao. Study on special electrode with high temperature corrosive resistance[J]. Transactions of the China Welding Institution, 2003, 24(6): 81-84. (in Chinese)
[6] XUE Hai-tao, LI Jian-guo, LI Jun-yue, et al. Mechanism of rare earth oxide transferring from coated electrode to welding pool[J]. Journal of the Chinese Rare Earth Society, 2003, 21(5): 580-583. (in Chinese)
[7] MICHELS H T. The effect of dispersed reactive metal oxides on the oxidation resistance of nickel-20 wt pet chromium alloys[J]. Metallurgical Transactions A, 1976, 7A(3): 379-386.
[8] WANG B, LIU M C, MENG Q S. A study of efficient e0-19-10-16 stainless steel electrode[J]. Journal of Taiyuan University of Technology, 1991, 22(2): 1-6. (in Chinese)
[9] SU Qiang. Rare Earth Chemical[M]. Zhengzhou: Henan Science & Technology Press, 1993. (in Chinese)
[10] DU Ting, HAN Qi-yong, WANG Chang-zhen. The Physical Chemistry of Appication of Rare Earth and Alkaline Earth and Their Applicationon Material[M]. Beijing: Science Press, 1995. (in Chinese)
[11] WANG Yan-qing. The effect of La on high temperature alloy[J]. Metal Abroad, 1984 (12): 26-30. (in Chinese)
(Edited by YANG You-ping)
Corresponding author: LI Zhi-yong; Tel: +86-351-3921389; Fax: +86-351-3557351; E-mail: lzhy_2002@126.com