
Interfacial electrokinetic characteristics before and after bioleaching microorganism adhesion to pyrite
LIU Jian-she(柳建设), WANG Zhao-hui(王兆慧), CHEN Hong(陈 红), ZHANG Yan-hua(张艳华)
School of Resources Processing and Bioengineering, Central South University, Changsha 410083, China
Received 28 July 2005; accepted 8 November 2005
Abstract: Zeta potentials of pyrite and Acidithiobacillus ferrooxidans cultured by sulfur in different levels of ionic strength and pH values were measured by Coulter Delsa 440SX zeta potential determinator. Meanwhile, the effects of bacterial adhesion and bacterial concentration on zeta potential of pyrite after adsorption were investigated. The results show that with the increase of ionic strength, zeta potentials of pyrite decrease in the range of pH 2.5-10.5 and the isoelectric point(IEP) of mineral shifts to the left. It is also found that the specific adsorption on pyrite of chloride ion can affect zeta potentials of pyrite sharply. As bacterial adsorption occurs, IEP of pyrite shifts towards that of Acidithiobacillus ferrooxidans; as bacterial concentration is increscent, this tendency is even larger and more obvious. Finally, a reasonable explanation for above-mentioned experimental phenomena was given by electrical double layer model and surface ionization model.
Key words: Acidithiobacillus ferrooxidans; pyrite; adhesion; electrical double layer model; zeta potential; isoelectric point
1 Introduction
Zeta potential, as a critical parameter of colloidal particles, has been widely used in many fields[1-3], such as material,beneficiation and biology. As measured by electro-osmosis, fluid stream electrophoresis and microelectrophoresis, zeta potential can contribute to the analysis for mechanics of interaction between solid particles and solution component. Therefore, researchers in bio-hydrometallurgy manage to obtain some information for surface electric property using zeta potential before and after bacterial adhesion to metal sulfides. As for aspects of bacterial zeta potential, it is proved that the Acidithiobacillus ferrooxidans(A.f) growing in different substrates are varied in zeta potential. DEVASIA et al[5] pointed out that cells cultured by both ferrous iron and iron thiosulfate have shown the similar electrokinetic phenomena, namely having the same IEP. However, it is indicated that surface chemical property of cell varied with its growth condition because IEP of cells growing in sulfur substrate is variable from about 2 to 3.8. With the metal sulfides, pyrite is the most extensively-studied object, whose IEP is commonly among 6-8[6] but with the exception[7]. It is reported that as bacterial adsorption occurs, IEP of pyrite shifts towards that of A.f, which suggests modification for mineral surface property after adsorption process[8].
However, zeta potential measurement will be affected by different experimental factors like ionic strength, electrolyte composition and pH. Unfortunately, these effects are often neglected thus leading to different experimental results. Moreover, in essence, zeta potential is a potential of sliding plane(or shear plane) in electrical double layer model for solid liquid interface. So the shortage of lacking theoretical confirmation for many results of zeta potential may be made well according to electrical double layer theory.
In this study, zeta potentials of pyrite and A.f cultured by sulfur in different levels of ionic strength and pH values were measured in order to contrastively discover the alternation of surface property modified by bacterial adhesion. At the same time, we tend to explain the experimental results by Gouy-Chapman-Stern model.
2 Experimental
2.1 Materials
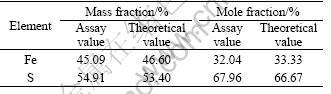
Pyrite used in experiment was well-crystallized mineral from domestic geological museum. Its chemical composition analysis by scanning electron probe is listed in Table 1. After ultrasonic treatment in order to remove surface oxide resulting from exposure to air, mineral particles were moved to agate mortar and well polished until their mesh size was up to <10 μm. The powder was sealed and reserved in wild-mouth bottle.
Table 1 Surface constituent of pyrite
A.f strain in the experiment was isolated and purified from drain water in Zhuzhou, China. Element sulfur was chosen as energy source just because of its advantage of less precipitate and higher biomass. The cultivation of A.f strain was identical to Ref.[9]. When growth of bacteria was in the logarithmic phase, the bacteria culture was first passed through Waterman No.44 filter paper to remove residual element sulfur. The filtrate was then centrifuged at 5 000 r/min for 30 min. The residual pellet was rinsed by double-distilled water and then centrifuged as described earlier. This procedure was repeated for three times to make sure the removal of all the precipitate. The cell pellet was finally suspended in distilled water and stored in a refrigerator at 4 ℃.
Sodium chloride (chromatographically grade) was used as supporting electrolyte in the measurement. pH adjusted agents were hydrochloric acid and sodium hydroxide(analytically pure). The solvent was double- distilled water all the way.
2.2 Equipment and apparatus
Equipment and apparatus used in this experiment: agate mortar, 0.1 mg analytical balance, electric magnetic stirring apparatus, ultrasonic cleaner (CQ50), precise standard pH meter (pHS-3C), Coulter Delsa Zeta potential apparatus.
2.3 Experimental method
Sodium chloride solution with a series of ionic strength equal to 0.001, 0.01, 0.1 and 1 mol/L were prepared. Then a concentration of 1 g/L for mineral and for bacteria was employed respectively. The mixture was agitated for 30 min in magnetic stirring apparatus for uniformity of the suspension. When the mineral concentration was maintained at 1 g/L, 2 mL and 10 mL(2×106 cells/mL) of bacterial suspension were added, respectively. After 3 h of agitation, mineral was rinsed by double-distilled water for three times so as to remove all the loosely-adhered bacteria. Finally, the mineral was put into sodium chloride solution of 0.01 mol/L. Note that pH values were adjusted with sodium hydroxide and hydrochloric acid in all the measurement. The zeta potentials at various pH values from 2.5 to 10.5 were measured with Coulter Delsa 440SX zeta potential determinator. Repeated measure- ments were done for three times assuming relative error was less than 5%. And then the results were obtained by average method.
3 Results and discussion
3.1 Prediction of interfacial electrokinetic characteri- stics before and after bioleaching microorganism adhesion to pyrite
3.1.1 Electrical double layer at pyrite/solution interface
After the treatment of crushing and polishing, crystal structure of minerals were broken. The outward atoms, ions and molecules of surface layer were suspended, yielding residual unsaturated energy[10]. While minerals are dispersed into solution, the particles with huge free enthalpy will selectively adsorb certain ions, especially ions with the same element, isomorphous ions and H+ or OH-. According to surface ionization model, such a series of reaction[11] on surface of pyrite will occur as described in Eqns.(1)-(4).
FeOH(surf)+H+= (surf) (1)
(surf) (1)
FeOH(surf)+OH-=FeO-(surf)+H2O (2)
FeSH(surf)+H+=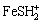 (surf) (3)
(surf) (3)
FeSH(surf)=FeS-(surf)+H+ (4)
There are counter-ions with opposite charge left in solution due to adsorption of two kinds of locating ions. The compact layer, known as adsorption layer as well, are generated while those counter-ions adsorb to particle tightly. In addition, the other fraction of counter-ions distribute in diffuse layer due to their thermal motion. Consequently, the electrical double layer structure at pyrite/solution interface is established.
From the previous model, it can be predicted that protonation reaction as described in Eqns.(1)-(3) on the surface of pyrite particle occurs under the acid condition, and the functional group (surf),
(surf), 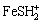 (surf) dominate on the surface thus mineral is positive-charged, while under the alkali condition, mineral is negative- charged caused by the dominating functional group FeO-(surf), FeS-(surf). Therefore, we can easily estimate that pyrite reaches its IEP in neutral case.
(surf) dominate on the surface thus mineral is positive-charged, while under the alkali condition, mineral is negative- charged caused by the dominating functional group FeO-(surf), FeS-(surf). Therefore, we can easily estimate that pyrite reaches its IEP in neutral case.
3.1.2 Effect of ionic strength on electrokinetic character- ristics
According to the classical Gouy-Chapman-Stern (GCS) electrical double layer model, the zeta potential can substitute for stern potential at low ionic strength. Therefore, effect of ionic strength on zeta potential can be explained with classical electrical double layer model [12].
In GCS model, diffuse layer can be taken equivalently as parallel-plate electric capacitor under the low ionic strength condition. So the expression of stern potential φ can be simplified as follows:
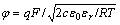 (5)
(5)
where c is the electrolyte concentration, and q is the surface charge density.
From the analysis above, it can be inferred that zeta potential decreases as diffuse layer is compressed with the increase of electrolyte concentration.
3.2 Effect of ionic strength on zeta potential of pyrite
0.1 g of pyrite powder was added into 100 mL NaCl solution with a series of ionic strength, i.e, 0.001, 0.01, 0.1 and 1 mol/L. Then the pH value was adjusted and the mixture was stirred to allow for the homogeneous dispersion. The results are represented graphically in Fig.1.
It can be seen from Fig.1(a), it seems abnormal that the measured zeta potential at 0.001 mol/L is always negative and absolute values of zeta potential increase with the increase of pH value. WANG et al[13] and ZUKOSKI et al[14] figured out that many electro- phoretic mobility data are definitely low due to effect of dynamical factors in the measurement, thus it fails to demonstrate the electrification of suspended particles objectively.
Fig.1(b) shows good coincidence with theoretical prediction: two curves at 0.01, 0.1 mol/L respectively make the similar performance, that is, absolute values of zeta potential go up firstly and down later. Meanwhile, at the same pH value, zeta potential at 0.1 mol/L is obviously larger than that at 0.01 mol/L. it is mostly because electrical double layer in pyrite/solution interface is compressed due to the increasing ionic strength. Consequently, IEP of pyrite at 0.01 mol/L is 7.2 while the value becomes 6.3 at 0.1 mol/L.
However, the same conclusion can not be drawn from Fig.1(c) at 1 mol/L. Absolute values of zeta potential under lower pH values decrease gradually, but when pH is around 7, the data increase sharply, and later go down continuously under the alkali condition. It is paradoxical to the explanation we made earlier. Why it happened? Note that chloride ion is an active ion in electrochemistry, specific adsorption may largely occur while its concentration is up to 1 mol/L at ionic strength of 1 mol/L. According to the triple layer model[15], the particles are positively-charged while residual charge in the solution is yielded by anion which can adsorb particles electro statically or specifically. As the charge from above mentioned adsorption exceeds surface charge of particles, the so-called “overload adsorption” happens. So the extra negative charge is about to absorb the cation around the particles thus forming a new structure as illustrated in Fig.2. It is very exciting that diffuse layer potential is opposite to the surface potential in sign. As represented in Fig.1(c), although pyrite should have been positively charged in the pH range of 2.5-6.5 according to surface ionization model, zeta potential becomes negative because of specific adsorption of chloride ion. With the increase of pH value, the fraction of electrostatic adsorption weakens as the amounts of functional groups  (surf),
(surf), 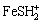 (surf) are decreased by degrees. Therefore the reduction absolute value of zeta potential is achieved. But the situation is different because pyrite is considered negatively- charged particle among pH 7.5-10.5. Due to electrostatic repulsion, the parts of specific adsorption escape from particles, so the decrease of data goes on.
(surf) are decreased by degrees. Therefore the reduction absolute value of zeta potential is achieved. But the situation is different because pyrite is considered negatively- charged particle among pH 7.5-10.5. Due to electrostatic repulsion, the parts of specific adsorption escape from particles, so the decrease of data goes on.

Fig.1 Relationship between zeta potential of pyrite and pH value at different concentrations: (a) 0.001 mol/L; (b) 0.1 mol/L, 0.01 mol/L; (c) 1.0 mol/L
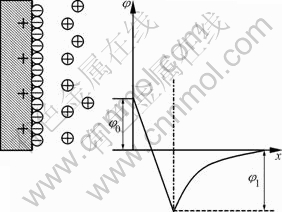
Fig.2 Triple electric layer structure and potential profile (φ0 is surface potential; φ1 is diffuse layer potential, i.e. zeta potential)
3.3 Electrokinetic characteristics of A.f cultured by sulfur
A.f is classified as Gram-negative bacteria. Its cell wall is composed of lipopolysaccharide, lipoprotein and surface protein. Bacterial cell surface charge originates from dissociation or protonation of carboxyl, amino groups and hydroxyl group[16]. Consequently, IEP of bacteria indicates the acid-base balance between anions and cations. Fig.3 represents the relation between zeta potential of A.f and pH value at 1.0 mol/L. IEP for A.f equal to 3.8 can be calculated from this graph, which shows a good agreement with Ref.[5]. DEVASIA et al [17] found that the amount of protein on the surface of bacteria growing in solid substrate is far larger than that of growing in liquid. What is more, after the removal of these proteins by proteinase K, the cells growing in both solid and liquid show similar manners in electrokinetic characteristics. As a result, protein content on the surface of bacteria really affects electrokinetic behavior of bacteria. It is probable because large amount of protonation of amino group results in being positively charged of bacteria below 3.8 of pH.
3.4 Effect of A.f adhesion on zeta potential of pyrite
Due to the hydrophobic and electrostatic interaction between A.f and mineral, bacterial cells will selectively adhere to the sites with high surface energy on the surface of mineral thus bringing about the fall of Gibbs free energy of mineral. The functional groups including carboxyl, aminos and hydroxyl groups cause the result that bacteria are charged, and inevitably influenced the mineral’s electric property after bacterial adhesion. This effect is apparently illustrated in Fig.4, that is, as bacteria content is enhanced, IEP of pyrite varies from 7.2 in the absence of bacteria to 5.3 and 4.2 in the case of inoculation respectively.

Fig.3 Relationship between zeta potential of A.f and pH value at 1.0 mol/L

Fig.4 Relationship between zeta potential of pyrite after bacteria with different bacterial concentrations adhering to mineral: a 2 mL of bacteria suspension added; b 10 mL of bacteria suspension added; c Sterile
In response to Gouy-Chapman electrical double layer model, the equations of surface charge density as a function of surface potential and the variation of diffuse layer potential with surface layer can be formulated as follows:
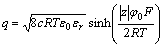 (6)
(6)
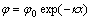 (7)
(7)
where q is the surface charge density of particle, φ0 indicates the surface potential of particle, φ is the potential at the position of x away from surface; κ is the reciprocal of Debye length.
Because bacteria are opposite to mineral in electric property, surface charge density should go down after bacterial adsorption. It can be inferred from Eqn.(6) that, surface potential of particle will be correspondingly decreased. Moreover, as illustrated in Fig.5, bacterial adsorption may cause the magnification of the diameter of pyrite particles, which may further make the shear plane travel towards the direction far from the surface. With the increasing distance x and the descending surface potential φ0, we can safely presume that bacterial adhesion finally leads to lowering of zeta potential of bacteria.

Fig.5 Schematic diagram of surface structure of pyrite after bacterial adhesion
4 Conclusions
1) Zeta potential of pyrite is obviously affected by ionic strength; however, freak result will be yielded in both higher and lower level of ionic strength. Under higher ionic strength, the negative zeta potential is made mostly because of overload adsorption of chloride ion. For moderate ionic strength of 0.01, 0.1 mol/L, IEP of pyrite is equal to 7.2 and 6.3 respectively. It can be interpreted with electrical double layer theory, that is, the increasing ionic strength will cause compression of electrical double layer whereby decreasing zeta potential of mineral.
2) For A.f cultured by sulfur, IEP is 3.8. Protein content on the surface of bacteria will contribute to the fluctuation of zeta potential.
3) As bacterial adsorption occurs, IEP of pyrite shifts towards that of A.f; as bacterial concentration is increscent, this tendency is even larger and more obvious.
4) Electrical double layer model and surface ionization model can explain experimental results above successfully and qualitatively, a novel manner for the study of electrokinetic characteristics of minerals is provided.
References
[1] SONG Xiao-lan, WANG Hai-bo, WU Xue-lan, QU Peng, QIU Guan-zhou. Synthesis of γ-Al2O3 with CeO2 nanoparticle and study on dispersion and stability for its suspension [J]. Journal of the Chinese Rare Earth Society, 2004, 22(6): 800-805.
[2] WU Bo-zeng, WANG Dian-zuo, QIU Guan-zhou, QIN Wen-qing. The aggregation and flocculation of ultrafine cassiterite in hydro- phobic system [J]. J Cent South Univ Technol, 2001, 32(3): 239-242.
[3] DITTRICH M, SIBLER S. Cell surface groups of two picocyanobacteria strains studied by zeta potential investigations, potentiometric titration and infrared spectroscopy [J]. Journal of Colloid and Interface Science, 2005, 286: 487-495.
[4] SHARMA P K, RAO K H. Adhesion of Paenibacillus polymyxa on chalcopyrite and pyrite: surface thermodynamics and extended DLVO theory [J]. Colloids and Surfaces B: Biointerfaces, 2003, 29: 21-38.
[5] DEVASIA P, NATARAJAN K A, SATHYANARAYANA D N, RAMANANDA RAO G. Surface chemistry of Thiobacillus ferrooxidans relevant to adhesion on mineral surfaces [J]. Applied and Environmental Microbiology, 1993, 59(12): 4051-4055.
[6] JIANG C L, WANG X H, PAREKH B K, LEONARD J W. The surface and solution chemistry flotation with xanthate in the presence of iron ions [J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 1998, 136: 51-62.
[7] BEBIE J, SCHOONEN M A A, FUHRMANN M, STRONGIN D R. Surface charge development on transition metal sulfides: An electrokinetic study [J]. Geochimica et Cosmochimica Acta, 1998, 62(4): 633-642.
[8] SHARMA P K, HANUMANTH R K, NATARAJA K, FORSSBERG K S E. Bioflotation of sulfide minerals in the presence of heterotrophic and chemolithotrophic bacteria [J]. Metallic Ore Dressing Abroad, 2001, 2: 37-42.
[9] ZHANG Jun, FAN Wei-ping, FANG Ping. Effect of substrates on bio-oxidation catalyzed by T. ferrooxidans [J]. Journal of Nanjing University of Chemical Technology, 2001, 23(6): 37-41.
[10] LU Long, LEI Liang-cheng, LIN Jin-fu, ZHANG Pei-hua. Study on the physical and reactive nature of mineral surfaces: advances and applications [J]. Journal of Guilin Institute of Technology, 2002, 22(3): 354-358.
[11] VALDIVIESO A L, CERVANTES T C, SONG S, CABRERA A R, LASKOWSKI J S. Dextrin as a non-toxic depressant for pyrite in flotation with xanthates as collector [J]. Minerals Engineering, 2004, 17: 1001-1006.
[12] YANG Song-qing, GONG Zhu-qing. Theoretical Electrochemistry [M]. Changsha: Central South University of Technology Press, 1996. 109-110.
[13] WANG Bao-dong, LIU Feng, ZHAN Run. A method of determining the stern potential of estuarine suspended particles [J]. Journal of Oceanography of Huanghai & Bohai Seas, 2000, 8(2): 73-78.
[14] ZUKOSKI C F, SAVILLE D A. The interpretation of electrokinetic measurement using a dynamic model of the stern layer [J]. Journal of Colloid and Interface Science, 1986, 114(1): 32-53.
[15] CHA Quan-xing. Introduction of Electrode Kinetics (2nd ed) [M]. Beijing: Science Press, 1987. 44-45.
[16] ALBERT T P, ROLF B, WILLEM N, et al. Electric double layer interactions in bacterial adhesion to surfaces [J]. Surface Science Reports, 2002, 47: 1-32.
[17] DEVASIA P, NATARAJAN K A, RAO G R. Role of bacterial growth conditions and adhesion in bioleaching of chalcopyrite by Thiobacillus ferrooxidans [J]. Metallic Ore Dressing Abroad, 1999, 2: 28-30.
Foundation item: Project(50374075) supported by the National Natural Science Foundation of China; Project(2004CB619204) supported by the National Basic Research Priorities Program of China
Corresponding author: LIU Jian-she; Tel: +86-731-8836372; E-mail: ljscsu@263.net
(Edited by LI Xiang-qun)